T cell receptor signals enhance susceptibility to Fas-mediated apoptosis
- PMID: 9382892
- PMCID: PMC2199157
- DOI: 10.1084/jem.186.11.1939
T cell receptor signals enhance susceptibility to Fas-mediated apoptosis
Abstract
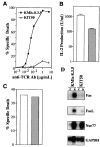
Fas(CD95) and its ligand (FasL) interaction plays a pivotal role in T cell receptor (TCR)-mediated apoptosis. However, the susceptibility of T cells to Fas-mediated apoptosis is tightly regulated during immune responses, a regulation which is thought to maintain the antigen-specificity of T cell apoptosis. Here we show that TCR stimulation enhances the induction of Fas-mediated apoptosis. In addition, using a mutant T cell hybridoma with impaired FasL expression, we show that the synergy provided by TCR stimulation can be mimicked by activators of PKC but not calcium influx. This effect cannot be inhibited by actinomycin D, suggesting that TCR stimulation leads to the alteration in preexisting signaling molecules to enhance Fas-mediated apoptosis. Our results therefore provide a mechanism of how Fas-FasL interactions lead to T cell death in an antigen-specific manner via repetitive antigen stimulation.
Figures


References
-
- von Boehmer H. Positive selection of lymphocytes. Cell. 1994;76:219–228. - PubMed
-
- Nossal GJV. Negative selection of lymphocytes. Cell. 1994;76:229–239. - PubMed
-
- Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. - PubMed
-
- Lynch DH, Ramsdell F, Alderson MR. Fas and FasL in the homeostatic regulation of immune responses. Immunol Today. 1995;16:569–574. - PubMed
-
- van Parijs L, Abbas AK. Role of Fas-mediated cell death in the regulation of immune responses. Curr Opin Immunol. 1996;8:355–361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

