Regulation of KATP channel activity by diazoxide and MgADP. Distinct functions of the two nucleotide binding folds of the sulfonylurea receptor
- PMID: 9382893
- PMCID: PMC2229399
- DOI: 10.1085/jgp.110.6.643
Regulation of KATP channel activity by diazoxide and MgADP. Distinct functions of the two nucleotide binding folds of the sulfonylurea receptor
Abstract
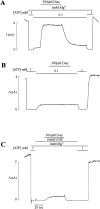
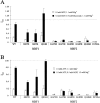
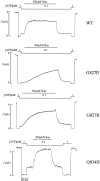
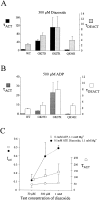
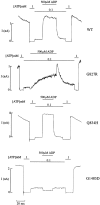
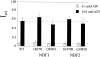
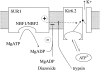
KATP channels were reconstituted in COSm6 cells by coexpression of the sulfonylurea receptor SUR1 and the inward rectifier potassium channel Kir6.2. The role of the two nucleotide binding folds of SUR1 in regulation of KATP channel activity by nucleotides and diazoxide was investigated. Mutations in the linker region and the Walker B motif (Walker, J.E., M.J. Saraste, M.J. Runswick, and N.J. Gay. 1982. EMBO [Eur. Mol. Biol. Organ.] J. 1:945-951) of the second nucleotide binding fold, including G1479D, G1479R, G1485D, G1485R, Q1486H, and D1506A, all abolished stimulation by MgADP and diazoxide, with the exception of G1479R, which showed a small stimulatory response to diazoxide. Analogous mutations in the first nucleotide binding fold, including G827D, G827R, and Q834H, were still stimulated by diazoxide and MgADP, but with altered kinetics compared with the wild-type channel. None of the mutations altered the sensitivity of the channel to inhibition by ATP4-. We propose a model in which SUR1 sensitizes the KATP channel to ATP inhibition, and nucleotide hydrolysis at the nucleotide binding folds blocks this effect. MgADP and diazoxide are proposed to stabilize this desensitized state of the channel, and mutations at the nucleotide binding folds alter the response of channels to MgADP and diazoxide by altering nucleotide hydrolysis rates or the coupling of hydrolysis to channel activation.
Figures



References
-
- Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JP, IV, Boyd AE, III, Gonzalez G, Herrera H, Sosa, Nguy K, Bryan J, Nelson DA. The β cell high affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–426. - PubMed
-
- Anderson MP, Welsh MJ. Regulation by ATP and ADP of CFTR chloride channels that contain mutant nucleotide-binding domains. Science. 1992;257:1701–1704. - PubMed
-
- Ashcroft FM. Adenosine 5′-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. - PubMed
-
- Baukrowitz T, Hwang TC, Nairn AC, Gadsby DC. Coupling of CFTR Cl−channel gating to an ATP hydrolysis cycle. Neuron. 1994;12:473–482. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

