Octameric stoichiometry of the KATP channel complex
- PMID: 9382894
- PMCID: PMC2229396
- DOI: 10.1085/jgp.110.6.655
Octameric stoichiometry of the KATP channel complex
Abstract
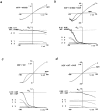
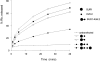
ATP-sensitive potassium (KATP) channels link cellular metabolism to electrical activity in nerve, muscle, and endocrine tissues. They are formed as a functional complex of two unrelated subunits-a member of the Kir inward rectifier potassium channel family, and a sulfonylurea receptor (SUR), a member of the ATP-binding cassette transporter family, which includes cystic fibrosis transmembrane conductance regulators and multidrug resistance protein, regulators of chloride channel activity. This recent discovery has brought together proteins from two very distinct superfamilies in a novel functional complex. The pancreatic KATP channel is probably formed specifically of Kir6.2 and SUR1 isoforms. The relationship between SUR1 and Kir6.2 must be determined to understand how SUR1 and Kir6.2 interact to form this unique channel. We have used mutant Kir6.2 subunits and dimeric (SUR1-Kir6.2) constructs to examine the functional stoichiometry of the KATP channel. The data indicate that the KATP channel pore is lined by four Kir6.2 subunits, and that each Kir6.2 subunit requires one SUR1 subunit to generate a functional channel in an octameric or tetradimeric structure.
Figures

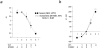
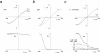
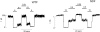

References
-
- Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JP, IV, Boyd AE, III, Gonzalez G, Herrera H, Sosa, Nguy K, Bryan J, Nelson DA. Cloning of the β-cell high affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–426. - PubMed
-
- Ammala C, Moorhouse A, Gribble F, Ashfield R, Proks P, Smith PA, Sakura H, Coles B, Ashcroft SJ, Ashcroft FM. Promiscuous coupling between the sulphonylurea receptor and inwardly rectifying potassium channels. Nature. 1996;379:545–548. - PubMed
-
- Ashcroft FM. Adenosine 5′-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. - PubMed
-
- Ciani S, Ribalet B. Ion permeation and rectification in ATP-sensitive channels from insulin-secreting cells (RINm5F): effects of K+, Na+ and Mg2+ . J Membr Biol. 1988;103:171–180. - PubMed
-
- Clement JP, IV, Kunjilwar K, Gonzalez G, Schwanstecher M, Panten U, Aguilar-Bryan L, Bryan J. Association and stoichiometry of KATPchannel subunits. Neuron. 1997;18:827–838. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

