Dual role of the Smad4/DPC4 tumor suppressor in TGFbeta-inducible transcriptional complexes
- PMID: 9389648
- PMCID: PMC316747
- DOI: 10.1101/gad.11.23.3157
Dual role of the Smad4/DPC4 tumor suppressor in TGFbeta-inducible transcriptional complexes
Abstract
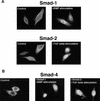
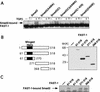
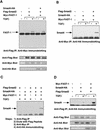
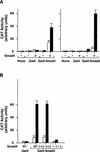
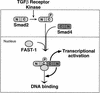
Upon ligand binding, the receptors of the TGFbeta family phosphorylate Smad proteins, which then move into the nucleus where they activate transcription. To carry out this function, the receptor-activated Smads 1 and 2 require association with the product of deleted in pancreatic carcinoma, locus 4 (DPC4), Smad4. We investigated the step at which Smad4 is required for transcriptional activation. Smad4 is not required for nuclear translocation of Smads 1 or 2, or for association of Smad2 with a DNA binding partner, the winged helix protein FAST-1. Receptor-activated Smad2 takes Smad4 into the nucleus where they form a complex with FAST-1 that requires these three components to activate transcription. Smad4 contributes two functions: Through its amino-terminal domain, Smad4 promotes binding of the Smad2/Smad4/FAST-1 complex to DNA; through its carboxy-terminal domain, Smad4 provides an activation function required for Smad1 or Smad2 to stimulate transcription. The dual function of Smad4 in transcriptional activation underscores its central role in TGFbeta signaling.
Figures


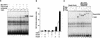
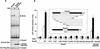
References
-
- Baker J, Harland RM. A novel mesoderm inducer, mMadr-2, functions in the activin signal transduction pathway. Genes & Dev. 1996;10:1880–1889. - PubMed
-
- Barrett MT, Schutte M, Kern SE, Reid BJ. Allelic loss and mutational analysis of the DPC4 gene in esophageal adenocarcinoma. Cancer Res. 1996;56:4351–4353. - PubMed
-
- Chen X, Rubock MJ, Whitman M. A transcriptional partner of MAD proteins in TGF-β signaling. Nature. 1996;383:691–696. - PubMed
-
- Chen X, Weisberg E, Fridmacher V, Watanabe M, Naco G, Whitman M. Smad4 and FAST-1 in the assembly of activin-responsive factor. Nature. 1997;389:85–89. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
