Mediation of NGF signaling by post-translational inhibition of HES-1, a basic helix-loop-helix repressor of neuronal differentiation
- PMID: 9389649
- PMCID: PMC316755
- DOI: 10.1101/gad.11.23.3168
Mediation of NGF signaling by post-translational inhibition of HES-1, a basic helix-loop-helix repressor of neuronal differentiation
Abstract
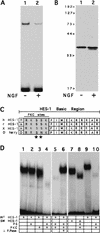
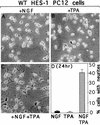
The induction of neurite outgrowth by NGF is a transcription-dependent process in PC12 cells, but the transcription factors that mediate this process are not known. Here we show that the bHLH transcriptional repressor HES-1 is a mediator of this process. Inactivation of endogenous HES-1 by forced expression of a dominant-negative protein induces neurite outgrowth in the absence of NGF and increases response to NGF. In contrast, expression of additional wild-type HES-1 protein represses and delays response to NGF. Endogenous HES-1 DNA-binding activity is post-translationally inhibited during NGF signaling in vivo, and phosphorylation of PKC consensus sites in the HES-1 DNA-binding domain inhibits DNA binding by purified HES-1 in vitro. Mutation of these sites generates a constitutively active protein that strongly and persistently blocks response to NGF. These results suggest that post-translational inhibition of HES-1 is both essential for and partially mediates the induction of neurite outgrowth by NGF signaling.
Figures







References
-
- Ausubel FM, Brent R, Kingsston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. second ed. New York, NY: Greene Publishing Associates and John Wiley & Sons; 1988.
-
- Bar-Sagi D, Feramisco JR. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell. 1985;42:841–848. - PubMed
-
- Benezra R, Davis RL, Lockshon D, Turner DL, Weintraub H. The protein Id: A negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990;61:49–59. - PubMed
-
- Borgatti P, Mazzoni M, Carini C, Neri LM, Marchisio M, Bertolaso L, Previati M, Zauli G, Capitani S. Changes of nuclear protein kinase C activity and isotype composition in PC12 cell proliferation and differentiation. Exp Cell Res. 1996;224:72–78. - PubMed
-
- Burstein DE, Blumberg PM, Greene LA. Nerve growth factor-induced neuronal differentiation of PC12 pheochromocytoma cells: Lack of inhibition by a tumor promoter. Brain Res. 1982;247:115–119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
