Hedgehog directly controls initiation and propagation of retinal differentiation in the Drosophila eye
- PMID: 9389656
- PMCID: PMC316756
- DOI: 10.1101/gad.11.23.3254
Hedgehog directly controls initiation and propagation of retinal differentiation in the Drosophila eye
Abstract
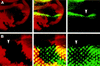
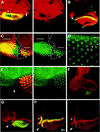
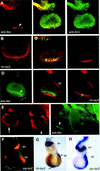
Patterning of the compound eye begins at the posterior edge of the eye imaginal disc and progresses anteriorly toward the disc margin. The advancing front of ommatidial differentiation is marked by the morphogenetic furrow (MF). Here we show by clonal analysis that Hedgehog (Hh), secreted from two distinct populations of cells has two distinct functions: It was well documented that Hh expression in the differentiating photoreceptor cells drives the morphogenetic furrow. Now we show that, in addition, Hh, secreted from cells at the posterior disc margin, is absolutely required for the initiation of patterning and predisposes ommatidial precursor cells to enter ommatidial assembly later. These two functions of Hh in eye patterning are similar to the biphasic requirement for Sonic Hh in patterning of the ventral neural tube in vertebrates. We show further that Hh induces ommatidial development in the absence of its secondary signals Wingless (Wg) and Dpp and that the primary function of Dpp in MF initiation is the repression of wg, which prevents ommatidial differentiation. Our results show that the regulatory relationships between Hh, Dpp, and Wg in the eye are similar to those found in other imaginal discs such as the leg disc despite obvious differences in their modes of development.
Figures

References
-
- Basler K, Struhl G. Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature. 1994;368:208–214. - PubMed
-
- Blackman RK, Sanicola M, Raftery LA, Gillevet T, Gelbart WM. An extensive 3′ cis-regulatory region directs the imaginal disk expression of decapentaplegic, a member of the TGF-beta family in Drosophila. Development. 1991;111:657–666. - PubMed
-
- Bonini NM, Choi KW. Early decisions in Drosophila eye morphogenesis. Curr Opin Genet Dev. 1995;5:507–515. - PubMed
-
- Bokor P, DiNardo S. The roles of hedgehog, wingless, and lines in patterning of the dorsal epidermis in Drosophila. Development. 1996;122:1083–1092. - PubMed
-
- Brook WJ, Cohen SM. Antagonistic interactions between Wingless and Decapentaplegic responsible for dorsal-ventral pattern in the Drosophila leg. Science. 1996;273:1373–1377. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
