Identification of two nervous system-specific members of the erg potassium channel gene family
- PMID: 9390998
- PMCID: PMC6573403
- DOI: 10.1523/JNEUROSCI.17-24-09423.1997
Identification of two nervous system-specific members of the erg potassium channel gene family
Abstract
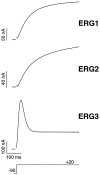
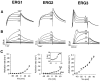
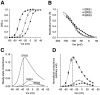
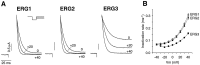
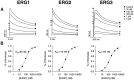
Two new potassium channel genes, erg2 and erg3, that are expressed in the nervous system of the rat were identified. These two genes form a small gene family with the previously described erg1 (HERG) gene. The erg2 and erg3 genes are expressed exclusively in the nervous system, in marked contrast to erg1, which is expressed in both neural and non-neural tissues. All three genes are expressed in peripheral sympathetic ganglia. The erg3 channel produces a current that has a large transient component at positive potentials, whereas the other two channels are slowly activating delayed rectifiers. Expression of the erg1 gene in the sympathetic nervous system has potential implications for the etiology of the LQT2 form of the human genetic disease long QT syndrome.
Figures



References
-
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. KVLQT1 and IsK (minK) proteins associate to form the IKs cardiac potassium current. Nature. 1996;384:78–80. - PubMed
-
- Brown DA. M currents. In: Narahashi T, editor. Ion channels. Plenum; New York: 1988. pp. 55–94. - PubMed
-
- Colman A. Translation of eukaryotic messenger RNA in Xenopus oocytes. In: Hames BD, Higgins SJ, editors. Transcription and translation. IRL; Oxford: 1984. pp. 271–302.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
