Regulation of ribonuclease III processing by double-helical sequence antideterminants
- PMID: 9391043
- PMCID: PMC28323
- DOI: 10.1073/pnas.94.25.13437
Regulation of ribonuclease III processing by double-helical sequence antideterminants
Abstract
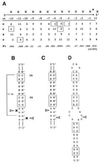
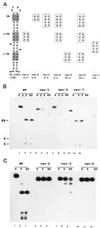
The double helix is a ubiquitous feature of RNA molecules and provides a target for nucleases involved in RNA maturation and decay. Escherichia coli ribonuclease III participates in maturation and decay pathways by site-specifically cleaving double-helical structures in cellular and viral RNAs. The site of cleavage can determine RNA functional activity and half-life and is specified in part by local tertiary structure elements such as internal loops. The involvement of base pair sequence in determining cleavage sites is unclear, because RNase III can efficiently degrade polymeric double-stranded RNAs of low sequence complexity. An alignment of RNase III substrates revealed an exclusion of specific Watson-Crick bp sequences at defined positions relative to the cleavage site. Inclusion of these "disfavored" sequences in a model substrate strongly inhibited cleavage in vitro by interfering with RNase III binding. Substrate cleavage also was inhibited by a 3-bp sequence from the selenocysteine-accepting tRNASec, which acts as an antideterminant of EF-Tu binding to tRNASec. The inhibitory bp sequences, together with local tertiary structure, can confer site specificity to cleavage of cellular and viral substrates without constraining the degradative action of RNase III on polymeric double-stranded RNA. Base pair antideterminants also may protect double-helical elements in other RNA molecules with essential functions.
Figures


References
-
- Belasco J G, Brawerman G, editors. Control of Messenger RNA Stability. San Diego: Academic; 1993.
-
- Nicholson A W. Prog Nucleic Acids Res Mol Biol. 1996;52:1–65. - PubMed
-
- Meegan J M, Marcus P I. Science. 1989;244:1089–1091. - PubMed
-
- Robertson H D, Webster R E, Zinder N D. J Biol Chem. 1968;243:82–91. - PubMed
-
- Court D. In: Control of Messenger RNA Stability. Belasco J G, Brawerman G, editors. San Diego: Academic; 1993. pp. 71–116.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

