Cell locomotion and focal adhesions are regulated by substrate flexibility
- PMID: 9391082
- PMCID: PMC28362
- DOI: 10.1073/pnas.94.25.13661
Cell locomotion and focal adhesions are regulated by substrate flexibility
Abstract
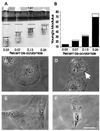
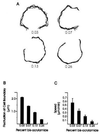
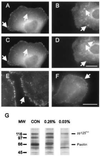
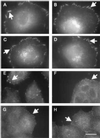
Responses of cells to mechanical properties of the adhesion substrate were examined by culturing normal rat kidney epithelial and 3T3 fibroblastic cells on a collagen-coated polyacrylamide substrate that allows the flexibility to be varied while maintaining a constant chemical environment. Compared with cells on rigid substrates, those on flexible substrates showed reduced spreading and increased rates of motility or lamellipodial activity. Microinjection of fluorescent vinculin indicated that focal adhesions on flexible substrates were irregularly shaped and highly dynamic whereas those on firm substrates had a normal morphology and were much more stable. Cells on flexible substrates also contained a reduced amount of phosphotyrosine at adhesion sites. Treatment of these cells with phenylarsine oxide, a tyrosine phosphatase inhibitor, induced the formation of normal, stable focal adhesions similar to those on firm substrates. Conversely, treatment of cells on firm substrates with myosin inhibitors 2,3-butanedione monoxime or KT5926 caused the reduction of both vinculin and phosphotyrosine at adhesion sites. These results demonstrate the ability of cells to survey the mechanical properties of their surrounding environment and suggest the possible involvement of both protein tyrosine phosphorylation and myosin-generated cortical forces in this process. Such response to physical parameters likely represents an important mechanism of cellular interaction with the surrounding environment within a complex organism.
Figures
References
-
- Bernstein L R, Liotta L A. Curr Opin Oncol. 1994;6:106–113. - PubMed
-
- Craig S W, Johnson R P. Curr Opin Cell Biol. 1996;8:74–85. - PubMed
-
- Palecek S P, Loftus J C, Ginsberg M H, Lauffenburger D A, Horowitz A F. Nature (London) 1997;385:537–540. - PubMed
-
- Wang N, Butler J P, Ingber D E. Science. 1993;260:1124–1127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

