Targeted expression of constitutively active receptors for parathyroid hormone and parathyroid hormone-related peptide delays endochondral bone formation and rescues mice that lack parathyroid hormone-related peptide
- PMID: 9391087
- PMCID: PMC28367
- DOI: 10.1073/pnas.94.25.13689
Targeted expression of constitutively active receptors for parathyroid hormone and parathyroid hormone-related peptide delays endochondral bone formation and rescues mice that lack parathyroid hormone-related peptide
Abstract
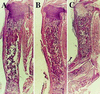
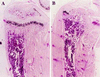
Mice in which the genes encoding the parathyroid hormone (PTH)-related peptide (PTHrP) or the PTH/PTHrP receptor have been ablated by homologous recombination show skeletal dysplasia due to accelerated endochondral bone formation, and die at birth or in utero, respectively. Skeletal abnormalities due to decelerated chondrocyte maturation are observed in transgenic mice where PTHrP expression is targeted to the growth plate, and in patients with Jansen metaphyseal chondrodysplasia, a rare genetic disorder caused by constitutively active PTH/PTHrP receptors. These and other findings thus indicate that PTHrP and its receptor are essential for chondrocyte differentiation. To further explore the role of the PTH/PTHrP receptor in this process, we generated transgenic mice in which expression of a constitutively active receptor, HKrk-H223R, was targeted to the growth plate by the rat alpha1 (II) collagen promoter. Two major goals were pursued: (i) to investigate how constitutively active PTH/PTHrP receptors affect the program of chondrocyte maturation; and (ii) to determine whether expression of the mutant receptor would correct the severe growth plate abnormalities of PTHrP-ablated mice (PTHrP-/-). The targeted expression of constitutively active PTH/PTHrP receptors led to delayed mineralization, decelerated conversion of proliferative chondrocytes into hypertrophic cells in skeletal segments that are formed by the endochondral process, and prolonged presence of hypertrophic chondrocytes with delay of vascular invasion. Furthermore, it corrected at birth the growth plate abnormalities of PTHrP-/- mice and allowed their prolonged survival. "Rescued" animals lacked tooth eruption and showed premature epiphyseal closure, indicating that both processes involve PTHrP. These findings suggest that rescued PTHrP-/- mice may gain considerable importance for studying the diverse, possibly tissue-specific role(s) of PTHrP in postnatal development.
Figures





References
-
- Erlebacher A, Filvaroff E H, Gitelman S E, Derynck R. Cell. 1995;80:371–378. - PubMed
-
- Jüppner H. Curr Opin Nephrol Hypertens. 1994;3:371–378. - PubMed
-
- Kronenberg H M, Bringhurst F R, Nussbaum S, Jüppner H, Abou-Samra A B, Segre G V, Potts J T., Jr . In: Handbook of Experimental Pharmacology: Physiology and Pharmacology of Bone. Mundy G R, Martin T J, editors. Heidelberg: Springer; 1993. pp. 185–201.
-
- Broadus A E, Stewart A F. In: The Parathyroids. Basic and Clinical Concepts. Bilzikian J P, Levine M A, Marcus R, editors. New York: Raven; 1994. pp. 259–294.
-
- Wysolmerski J J, McCaughern-Carucci J F, Daifotis A G, Broadus A E, Philbrick W M. Development (Cambridge, UK) 1995;121:3539–3547. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

