A family of genes required for maintenance of cell wall integrity and for the stress response in Saccharomyces cerevisiae
- PMID: 9391108
- PMCID: PMC28388
- DOI: 10.1073/pnas.94.25.13804
A family of genes required for maintenance of cell wall integrity and for the stress response in Saccharomyces cerevisiae
Abstract
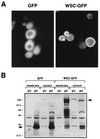
The PKC1-MPK1 pathway in yeast functions in the maintenance of cell wall integrity and in the stress response. We have identified a family of genes that are putative regulators of this pathway. WSC1, WSC2, and WSC3 encode predicted integral membrane proteins with a conserved cysteine motif and a WSC1-green fluorescence protein fusion protein localizes to the plasma membrane. Deletion of WSC results in phenotypes similar to mutants in the PKC1-MPK1 pathway and an increase in the activity of MPK1 upon a mild heat treatment is impaired in a wscDelta mutant. Genetic analysis places the function of WSC upstream of PKC1, suggesting that they play a role in its activation. We also find a genetic interaction between WSC and the RAS-cAMP pathway. The RAS-cAMP pathway is required for cell cycle progression and for the heat shock response. Overexpression of WSC suppresses the heat shock sensitivity of a strain in which RAS is hyperactivated and the heat shock sensitivity of a wscDelta strain is rescued by deletion of RAS2. The functional characteristics and cellular localization of WSC suggest that they may mediate intracellular responses to environmental stress in yeast.
Figures






Similar articles
-
Mutations in WSC genes for putative stress receptors result in sensitivity to multiple stress conditions and impairment of Rlm1-dependent gene expression in Saccharomyces cerevisiae.Mol Genet Genomics. 2001 Sep;266(1):142-55. doi: 10.1007/s004380100537. Mol Genet Genomics. 2001. PMID: 11589572
-
Saccharomyces cerevisiae heat shock transcription factor regulates cell wall remodeling in response to heat shock.Eukaryot Cell. 2005 Jun;4(6):1050-6. doi: 10.1128/EC.4.6.1050-1056.2005. Eukaryot Cell. 2005. PMID: 15947197 Free PMC article.
-
Saccharomyces cerevisiae mid2p is a potential cell wall stress sensor and upstream activator of the PKC1-MPK1 cell integrity pathway.J Bacteriol. 1999 Jun;181(11):3330-40. doi: 10.1128/JB.181.11.3330-3340.1999. J Bacteriol. 1999. PMID: 10348843 Free PMC article.
-
Interdependence of several heat shock gene activations, cyclic AMP decline and changes at the plasma membrane of Saccharomyces cerevisiae.Antonie Van Leeuwenhoek. 1990 Oct;58(3):195-201. doi: 10.1007/BF00548933. Antonie Van Leeuwenhoek. 1990. PMID: 2175162 Review. No abstract available.
-
Integrins in disguise - mechanosensors in Saccharomyces cerevisiae as functional integrin analogues.Microb Cell. 2019 Jul 15;6(8):335-355. doi: 10.15698/mic2019.08.686. Microb Cell. 2019. PMID: 31404395 Free PMC article. Review.
Cited by
-
Different selective pressures lead to different genomic outcomes as newly-formed hybrid yeasts evolve.BMC Evol Biol. 2012 Apr 2;12:46. doi: 10.1186/1471-2148-12-46. BMC Evol Biol. 2012. PMID: 22471618 Free PMC article.
-
Proteome of the nematode-trapping cells of the fungus Monacrosporium haptotylum.Appl Environ Microbiol. 2013 Aug;79(16):4993-5004. doi: 10.1128/AEM.01390-13. Epub 2013 Jun 14. Appl Environ Microbiol. 2013. PMID: 23770896 Free PMC article.
-
Functional and genetic interactions of TOR in the budding yeast Saccharomyces cerevisiae with myosin type II-deficiency (myo1Δ).BMC Cell Biol. 2012 May 30;13:13. doi: 10.1186/1471-2121-13-13. BMC Cell Biol. 2012. PMID: 22646158 Free PMC article.
-
Genetic interactions of yeast eukaryotic translation initiation factor 5A (eIF5A) reveal connections to poly(A)-binding protein and protein kinase C signaling.Genetics. 2002 Feb;160(2):393-405. doi: 10.1093/genetics/160.2.393. Genetics. 2002. PMID: 11861547 Free PMC article.
-
Cell Wall Integrity and Its Industrial Applications in Filamentous Fungi.J Fungi (Basel). 2022 Apr 23;8(5):435. doi: 10.3390/jof8050435. J Fungi (Basel). 2022. PMID: 35628691 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

