A point mutation in the MET oncogene abrogates metastasis without affecting transformation
- PMID: 9391119
- PMCID: PMC28399
- DOI: 10.1073/pnas.94.25.13868
A point mutation in the MET oncogene abrogates metastasis without affecting transformation
Abstract
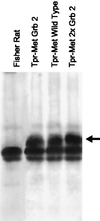
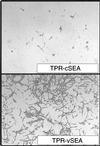
The MET oncogene encodes the tyrosine kinase receptor for hepatocyte growth factor/scatter factor (HGF), known to stimulate invasive growth of epithelial cells. MET is overexpressed in a significant percentage of human cancers and is amplified during the transition between primary tumors and metastasis. To investigate whether this oncogene is directly responsible for the acquisition of the metastatic phenotype, we exploited a single-hit oncogenic version of MET, able to transform and to confer invasive and metastatic properties to nontumorigenic cells, both in vitro and in nude mice. We mutagenized the signal transducer docking site of Met (Y1349VHVX3Y1356VNV), which has the uncommon property of binding and activating multiple src homology region 2 (SH2)-containing intracellular effectors. Notably, a point mutation (H1351 --> N) increased the transforming ability of the oncogene but abolished its metastatic potential. This mutation duplicates the Grb2 binding site, super-activating the Ras pathway and preventing the binding of the other intracellular transducers. Complementation in trans with another nonmetastatic mutant (N1358 --> H), recruiting all the transducers downstream to Met except Grb2, rescued the invasive-metastatic phenotype. It is concluded that the metastatic potential of the MET oncogene relies on the properties of its multifunctional docking site, and that a single point mutation affecting signal transduction can dissociate neoplastic transformation from metastasis.
Figures


References
-
- Liotta L A, Stetler-Stevenson W G. In: Cancer Principles and Practice of Oncology. 4th Ed. De Vita V Jr, Hellman S, Rosenberg S A, editors. Philadelphia: Lippincott; 1993. pp. 134–149.
-
- Egan S E, Wright J A, Jarolim L, Yanagihara K, Bassin R H, Greenberg A H. Science. 1987;238:202–205. - PubMed
-
- Yu D, Wang S, Dulski K M, Tsai C M, Nicolson G L, Hung M C. Cancer Res. 1994;54:3260–3266. - PubMed
-
- Comoglio P M, Boccaccio C. Genes to Cells. 1996;1:347–354. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

