Proliferation of adult T cell leukemia/lymphoma cells is associated with the constitutive activation of JAK/STAT proteins
- PMID: 9391124
- PMCID: PMC28404
- DOI: 10.1073/pnas.94.25.13897
Proliferation of adult T cell leukemia/lymphoma cells is associated with the constitutive activation of JAK/STAT proteins
Abstract
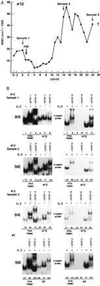
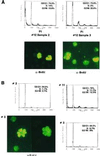
Human T cell leukemia/lymphotropic virus type I (HTLV-I) induces adult T cell leukemia/lymphoma (ATLL). The mechanism of HTLV-I oncogenesis in T cells remains partly elusive. In vitro, HTLV-I induces ligand-independent transformation of human CD4+ T cells, an event that correlates with acquisition of constitutive phosphorylation of Janus kinases (JAK) and signal transducers and activators of transcription (STAT) proteins. However, it is unclear whether the in vitro model of HTLV-I transformation has relevance to viral leukemogenesis in vivo. Here we tested the status of JAK/STAT phosphorylation and DNA-binding activity of STAT proteins in cell extracts of uncultured leukemic cells from 12 patients with ATLL by either DNA-binding assays, using DNA oligonucleotides specific for STAT-1 and STAT-3, STAT-5 and STAT-6 or, more directly, by immunoprecipitation and immunoblotting with anti-phosphotyrosine antibody for JAK and STAT proteins. Leukemic cells from 8 of 12 patients studied displayed constitutive DNA-binding activity of one or more STAT proteins, and the constitutive activation of the JAK/STAT pathway was found to persist over time in the 2 patients followed longitudinally. Furthermore, an association between JAK3 and STAT-1, STAT-3, and STAT-5 activation and cell-cycle progression was demonstrated by both propidium iodide staining and bromodeoxyuridine incorporation in cells of four patients tested. These results imply that JAK/STAT activation is associated with replication of leukemic cells and that therapeutic approaches aimed at JAK/STAT inhibition may be considered to halt neoplastic growth.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

