CENP-E function at kinetochores is essential for chromosome alignment
- PMID: 9396744
- PMCID: PMC2132614
- DOI: 10.1083/jcb.139.6.1373
CENP-E function at kinetochores is essential for chromosome alignment
Abstract
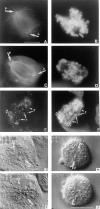
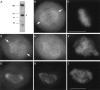
CENP-E is a kinesin-like protein that binds to kinetochores and may provide functions that are critical for normal chromosome motility during mitosis. To directly test the in vivo function of CENP-E, we microinjected affinity-purified antibodies to block the assembly of CENP-E onto kinetochores and then examined the behavior of these chromosomes. Chromosomes lacking CENP-E at their kinetochores consistently exhibited two types of defects that blocked their alignment at the spindle equator. Chromosomes positioned near a pole remained mono-oriented as they were unable to establish bipolar microtubule connections with the opposite pole. Chromosomes within the spindle established bipolar connections that supported oscillations and normal velocities of kinetochore movement between the poles, but these bipolar connections were defective because they failed to align the chromosomes into a metaphase plate. Overexpression of a mutant that lacked the amino-terminal 803 amino acids of CENP-E was found to saturate limiting binding sites on kinetochores and competitively blocked endogenous CENP-E from assembling onto kinetochores. Chromosomes saturated with the truncated CENP-E mutant were never found to be aligned but accumulated at the poles or were strewn within the spindle as was the case when cells were microinjected with CENP-E antibodies. As the motor domain was contained within the portion of CENP-E that was deleted, the chromosomal defect is likely attributed to the loss of motor function. The combined data show that CENP-E provides kinetochore functions that are essential for monopolar chromosomes to establish bipolar connections and for chromosomes with connections to both spindle poles to align at the spindle equator. Both of these events rely on activities that are provided by CENP-E's motor domain.
Figures






References
-
- Ault JG, Nicklas RB. Tension, microtubule rearrangements, and the proper distribution of chromosomes in mitosis. Chromosoma (Berl) 1989;98:33–39. - PubMed
-
- Cassimeris L, Rieder CL, Salmon ED. Microtubule assembly and kinetochore directional instability in vertebrate monopolar spindles: implications for the mechanism of chromosome congression. J Cell Sci. 1994;107:285–297. - PubMed
-
- Chalfie M, Tu Y, Euskirschen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1993;263:802–805. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

