Peroxisomal targeting, import, and assembly of alcohol oxidase in Pichia pastoris
- PMID: 9396748
- PMCID: PMC2132610
- DOI: 10.1083/jcb.139.6.1419
Peroxisomal targeting, import, and assembly of alcohol oxidase in Pichia pastoris
Abstract
Alcohol oxidase (AOX), the first enzyme in the yeast methanol utilization pathway is a homooctameric peroxisomal matrix protein. In peroxisome biogenesis-defective (pex) mutants of the yeast Pichia pastoris, AOX fails to assemble into active octamers and instead forms inactive cytoplasmic aggregates. The apparent inability of AOX to assemble in the cytoplasm contrasts with other peroxisomal proteins that are able to oligomerize before import. To further investigate the import of AOX, we first identified its peroxisomal targeting signal (PTS). We found that sequences essential for targeting AOX are primarily located within the four COOH-terminal amino acids of the protein leucine-alanine-arginine-phenylalanine COOH (LARF). To examine whether AOX can oligomerize before import, we coexpressed AOX without its PTS along with wild-type AOX and determined whether the mutant AOX could be coimported into peroxisomes. To identify the mutant form of AOX, the COOH-terminal LARF sequence of the protein was replaced with a hemagglutinin epitope tag (AOX-HA). Coexpression of AOX-HA with wild-type AOX (AOX-WT) did not result in an increase in the proportion of AOX-HA present in octameric active AOX, suggesting that newly synthesized AOX-HA cannot oligomerize with AOX-WT in the cytoplasm. Thus, AOX cannot initiate oligomerization in the cytoplasm, but must first be targeted to the organelle before assembly begins.
Figures



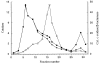
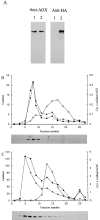
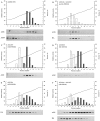


References
-
- Ausubel, F.M., R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith, and K. Struhl. 1996. Analysis of proteins. In Current Protocols in Molecular Biology. Vol. 2. John Wiley and Sons, New York. pp. 10.1–10.20.
-
- Bartel PL, Chien C-T, Sternglanz R, Fields S. Elimination of false positives that arise in using the two-hybrid system. Biotechniques. 1993;14:920–924. - PubMed
-
- Becker DM, Guarente L. High-efficiency transformation of yeast by electroporation. Methods Enzymol. 1991;194:182–187. - PubMed
-
- Bellion E, Goodman JM. Proton ionophores prevent assembly of a peroxisomal protein. Cell. 1987;48:165–173. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

