Muscle beta1D integrin reinforces the cytoskeleton-matrix link: modulation of integrin adhesive function by alternative splicing
- PMID: 9396762
- PMCID: PMC2132630
- DOI: 10.1083/jcb.139.6.1583
Muscle beta1D integrin reinforces the cytoskeleton-matrix link: modulation of integrin adhesive function by alternative splicing
Abstract
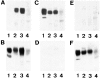
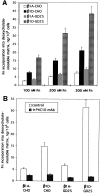
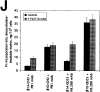
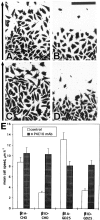
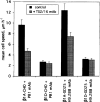
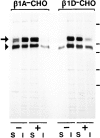
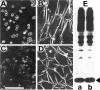
Expression of muscle-specific beta1D integrin with an alternatively spliced cytoplasmic domain in CHO and GD25, beta1 integrin-minus cells leads to their phenotypic conversion. beta1D-transfected nonmuscle cells display rounded morphology, lack of pseudopodial activity, retarded spreading, reduced migration, and significantly enhanced contractility compared with their beta1A-expressing counterparts. The transfected beta1D is targeted to focal adhesions and efficiently displaces the endogenous beta1A and alphavbeta3 integrins from the sites of cell-matrix contact. This displacement is observed on several types of extracellular matrix substrata and leads to elevated stability of focal adhesions in beta1D transfectants. Whereas a significant part of cellular beta1A integrin is extractable in digitonin, the majority of the transfected beta1D is digitonin-insoluble and is strongly associated with the detergent-insoluble cytoskeleton. Increased interaction of beta1D integrin with the actin cytoskeleton is consistent with and might be mediated by its enhanced binding to talin. In contrast, beta1A interacts more strongly with alpha-actinin, than beta1D. Inside-out driven activation of the beta1D ectodomain increases ligand binding and fibronectin matrix assembly by beta1D transfectants. Phenotypic effects of beta1D integrin expression in nonmuscle cells are due to its enhanced interactions with both cytoskeletal and extracellular ligands. They parallel the transitions that muscle cells undergo during differentiation. Modulation of beta1 integrin adhesive function by alternative splicing serves as a physiological mechanism reinforcing the cytoskeleton- matrix link in muscle cells. This reflects the major role for beta1D integrin in muscle, where extremely stable association is required for contraction.
Figures










References
-
- Altruda F, Cervella P, Tarone G, Botta C, Balzac F, Stefanuto G, Silengo L. A human integrin β1 subunit with a unique cytoplasmic domain generated by alternative mRNA processing. Gene. 1990;95:261–266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

