TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor
- PMID: 9396779
- PMCID: PMC2199171
- DOI: 10.1084/jem.186.12.2075
TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor
Abstract
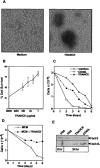
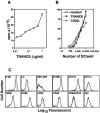
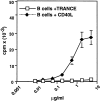
TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine) is a new member of the TNF family that is induced upon T cell receptor engagement and activates c-Jun N-terminal kinase (JNK) after interaction with its putative receptor (TRANCE-R). In addition, TRANCE expression is restricted to lymphoid organs and T cells. Here, we show that high levels of TRANCE-R are detected on mature dendritic cells (DCs) but not on freshly isolated B cells, T cells, or macrophages. Signaling by TRANCE-R appears to be dependent on TNF receptor-associated factor 2 (TRAF2), since JNK induction is impaired in cells from transgenic mice overexpressing a dominant negative TRAF2 protein. TRANCE inhibits apoptosis of mouse bone marrow-derived DCs and human monocyte-derived DCs in vitro. The resulting increase in DC survival is accompanied by a proportional increase in DC-mediated T cell proliferation in a mixed leukocyte reaction. TRANCE upregulates Bcl-xL expression, suggesting a potential mechanism for enhanced DC survival. TRANCE does not induce the proliferation of or increase the survival of T or B cells. Therefore, TRANCE is a new DC-restricted survival factor that mediates T cell-DC communication and may provide a tool to selectively enhance DC activity.
Figures
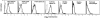
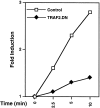
References
-
- Wyllie AH. Apoptosis and the regulation of cell numbers in normal and neoplastic tissues: an overview. Cancer Metastasis Rev. 1992;11:95–103. - PubMed
-
- Russell JH. Activation-induced death of mature T cells in the regulation of immune responses. Curr Opin Immunol. 1995;7:382–388. - PubMed
-
- Lynch DH, Ramsdell F, Alderson MR. Fas and FasL in the homeostatic regulation of immune responses. Immunol Today. 1995;16:569–574. - PubMed
-
- Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–962. - PubMed
-
- Rothe M, Wong SC, Henzel WJ, Goeddel DV. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell. 1994;78:681–692. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

