Defining extracellular integrin alpha-chain sites that affect cell adhesion and adhesion strengthening without altering soluble ligand binding
- PMID: 9398682
- PMCID: PMC25734
- DOI: 10.1091/mbc.8.12.2647
Defining extracellular integrin alpha-chain sites that affect cell adhesion and adhesion strengthening without altering soluble ligand binding
Abstract
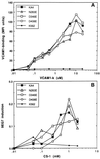
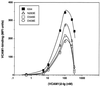
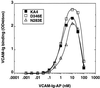
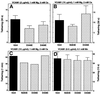
It was previously shown that mutations of integrin alpha4 chain sites, within putative EF-hand-type divalent cation-binding domains, each caused a marked reduction in alpha4beta1-dependent cell adhesion. Some reports have suggested that alpha-chain "EF-hand" sites may interact directly with ligands. However, we show here that mutations of three different alpha4 "EF-hand" sites each had no effect on binding of soluble monovalent or bivalent vascular cell adhesion molecule 1 whether measured indirectly or directly. Furthermore, these mutations had minimal effect on alpha4beta1-dependent cell tethering to vascular cell adhesion molecule 1 under shear. However, EF-hand mutants did show severe impairments in cellular resistance to detachment under shear flow. Thus, mutation of integrin alpha4 "EF-hand-like" sites may impair 1) static cell adhesion and 2) adhesion strengthening under shear flow by a mechanism that does not involve alterations of initial ligand binding.
Figures



References
-
- Alon R, Hammer DA, Springer TA. Lifetime of the P-selectin-carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature. 1995a;374:539–542. - PubMed
-
- Babu A, Su H, Ryu Y, Gulati J. Determination of residue specificity in the EF-hand of troponin C for Ca2+ coordination, by genetic engineering. J Biol Chem. 1992;267:15469–15474. - PubMed
-
- Bajt ML, Goodman T, McGuire SL. β2 (CD18) mutations abolish ligand recognition by I domain integrins LFA-1 (αLβ2, CD11a/CD18) and MAC-1 (αMβ2, CD11b/CD18) J Biol Chem. 1995;270:94–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

