Identification and characterization of a human protein kinase related to budding yeast Cdc7p
- PMID: 9405610
- PMCID: PMC24960
- DOI: 10.1073/pnas.94.26.14320
Identification and characterization of a human protein kinase related to budding yeast Cdc7p
Abstract
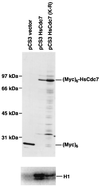
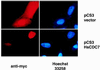
The Cdc7p protein kinase is essential for the G1/S transition and initiation of DNA replication during the cell division cycle in Saccharomyces cerevisiae. Cdc7p appears to be an evolutionarily conserved protein, since a homolog Hsk1 has been isolated from Schizosaccharomyces pombe. Here, we report the isolation of a human cDNA, HsCdc7, whose product is closely related in sequence to Cdc7p and Hsk1. The HsCdc7 cDNA encodes a protein of 574 amino acids with predicted size of 64 kDa. HsCdc7 contains the conserved subdomains common to all protein-serine/threonine kinases and three "kinase inserts" that are characteristic of Cdc7p and Hsk1. Immune complexes of HsCdc7 from cell lysates were able to phosphorylate histone H1 in vitro. Indirect immunofluorescence staining demonstrated that HsCdc7 protein was predominantly localized in the nucleus. Although the expression levels of HsCdc7 appeared to be constant throughout the cell cycle, the protein kinase activity of HsCdc7 increased during S phase of the cell cycle at approximately the same time as that of Cdk2. These results, together with the functions of Cdc7p in yeast, suggest that HsCdc7 may phosphorylate critical substrate(s) that regulate the G1/S phase transition and/or DNA replication in mammalian cells.
Figures


References
-
- Bell S P, Stillman B. Nature (London) 1992;357:128–134. - PubMed
-
- Diffley J F, Cocker J H. Nature (London) 1992;357:169–172. - PubMed
-
- Li J J, Herskowitz I. Science. 1993;262:1870–1874. - PubMed
-
- Micklem G, Rowley A, Harwood J, Nasmyth K, Diffley J F. Nature (London) 1993;366:87–89. - PubMed
-
- Liang C, Weinreich M, Stillman B. Cell. 1995;81:667–676. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

