Flickering fusion pores comparable with initial exocytotic pores occur in protein-free phospholipid bilayers
- PMID: 9405628
- PMCID: PMC25008
- DOI: 10.1073/pnas.94.26.14423
Flickering fusion pores comparable with initial exocytotic pores occur in protein-free phospholipid bilayers
Abstract
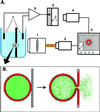
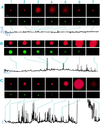
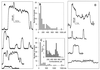
For the act of membrane fusion, there are two competing, mutually exclusive molecular models that differ in the structure of the initial pore, the pathway for ionic continuity between formerly separated volumes. Because biological "fusion pores" can be as small as ionic channels or gap junctions, one model posits a proteinaceous initial fusion pore. Because biological fusion pore conductance varies widely, another model proposes a lipidic initial pore. We have found pore opening and flickering during the fusion of protein-free phospholipid vesicles with planar phospholipid bilayers. Fusion pore formation appears to follow the coalescence of contacting monolayers to create a zone of hemifusion where continuity between the two adherent membranes is lipidic, but not aqueous. Hypotonic stress, causing tension in the vesicle membrane, promotes complete fusion. Pores closed soon after opening (flickering), and the distribution of fusion pore conductance appears similar to the distribution of initial fusion pores in biological fusion. Because small flickering pores can form in the absence of protein, the existence of small pores in biological fusion cannot be an argument in support of models based on proteinaceous pores. Rather, these results support the model of a lipidic fusion pore developing within a hemifused contact site.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

