Otogelin: a glycoprotein specific to the acellular membranes of the inner ear
- PMID: 9405633
- PMCID: PMC25017
- DOI: 10.1073/pnas.94.26.14450
Otogelin: a glycoprotein specific to the acellular membranes of the inner ear
Abstract
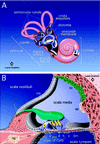
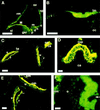
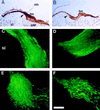
Efforts to identify the specific components of the mammalian inner ear have been hampered by the small number of neuroepithelial cells and the variety of supporting cells. To circumvent these difficulties, we used a PCR-based subtractive method on cDNA from 2-day-old mouse cochlea. A cDNA encoding a predicted 2910-amino acid protein related to mucin has been isolated. Several lines of evidence indicate, however, that this protein does not undergo the O-glycosylation characteristic to mucins. As confirmed by immunocytochemistry and biochemical experiments, this protein is specific to the inner ear. Immunohistofluorescence labeling showed that this protein is a component of all the acellular membranes of the inner ear: i.e., the tectorial membrane of the cochlea, the otoconial and accessory membranes of the utricule and saccule, the cupula of the semicircular canals, and a previously undescribed acellular material covering the otoconia of the saccule. The protein has been named otogelin with reference to its localization. A variety of nonsensory cells located underneath these membranes could be identified as synthesizing otogelin. Finally, this study revealed a maturation process of the tectorial membrane, as evidenced by the progressive organization of otogelin labeling into thick and spaced radial fiber-like structures.
Figures


References
-
- Hudspeth A J. Nature (London) 1989;341:397–404. - PubMed
-
- Hudspeth A J, Gillepsie P G. Neuron. 1994;12:1–9. - PubMed
-
- Denk W, Holt J R, Sheperd G M G, Corey D P. Neuron. 1995;15:1311–1321. - PubMed
-
- Lim D L, Anniko M. Acta Otolaryngol Suppl. 1985;42:1–69. - PubMed
-
- Tilney L G, Tilney M S, Saunders J S, DeRosier D J. J Cell Biol. 1986;106:355–365.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

