Receptors induce chemotaxis by releasing the betagamma subunit of Gi, not by activating Gq or Gs
- PMID: 9405640
- PMCID: PMC25031
- DOI: 10.1073/pnas.94.26.14489
Receptors induce chemotaxis by releasing the betagamma subunit of Gi, not by activating Gq or Gs
Abstract
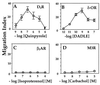
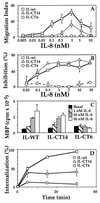
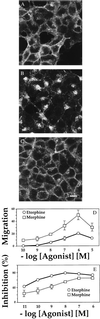
Many chemoattractants cause chemotaxis of leukocytes by stimulating a structurally distinct class of G protein-coupled receptors. To identify receptor functions required for chemotaxis, we studied chemotaxis in HEK293 cells transfected with receptors for nonchemokine ligands or for interleukin 8 (IL-8), a classical chemokine. In gradients of the appropriate agonist, three nonchemokine Gi-coupled receptors (the D2 dopamine receptor and opioid mu and delta receptors) mediated chemotaxis; the beta2-adrenoreceptor and the M3-muscarinic receptor, which couple respectively to Gs and Gq, did not mediate chemotaxis. A mutation deleting 31 C-terminal amino acids from the IL-8 receptor type B quantitatively impaired chemotaxis and agonist-induced receptor internalization, but not inhibition of adenylyl cyclase or stimulation of mitogen-activated protein kinase. To probe the possible relation between receptor internalization and chemotaxis, we used two agonists of the mu-opioid receptor. Morphine and etorphine elicited quantitatively similar chemotaxis, but only etorphine induced receptor internalization. Overexpression of two betagamma sequestering proteins (betaARK-ct and alphat) prevented IL-8 receptor type B-mediated chemotaxis but did not affect inhibition of adenylyl cyclase by IL-8. We conclude that: (i) Nonchemokine Gi-coupled receptors can mediate chemotaxis. (ii) Gi activation is necessary but probably not sufficient for chemotaxis. (iii) Chemotaxis does not require receptor internalization. (iv) Chemotaxis requires the release of free betagamma subunits.
Figures

References
-
- Baggiolini M, Dewald B, Moser B. Adv Immunol. 1994;55:97–179. - PubMed
-
- Murphy P M. Annu Rev Immunol. 1994;12:593–633. - PubMed
-
- Premack B A, Schall T J. Nat Med. 1996;2:1174–1178. - PubMed
-
- Gerard C, Gerard N P. Curr Opin Immunol. 1994;6:140–145. - PubMed
-
- Freedman N J, Lefkowitz R J. Recent Prog Horm Res. 1996;51:319–351. ; discussion, 352–353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

