DNA immunization: induction of higher avidity antibody and effect of route on T cell cytotoxicity
- PMID: 9405663
- PMCID: PMC25074
- DOI: 10.1073/pnas.94.26.14626
DNA immunization: induction of higher avidity antibody and effect of route on T cell cytotoxicity
Abstract
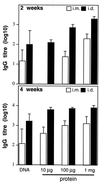
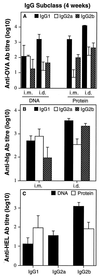
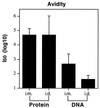
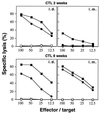
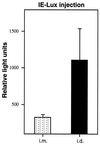
Immunizations of mice with plasmid DNAs encoding ovalbumin (OVA), human Ig, and hen egg lysozyme were compared with doses of soluble protein (without adjuvant) that induced similar IgG responses. The route of immunization influenced the magnitude of the antibody (Ab) response in that intradermal (i.d.) injection elicited higher IgG Ab levels than i.m. injection in both DNA- and protein-immunized mice. Although total IgG levels were similar to soluble protein controls, the avidity of the anti-OVA Abs generated by DNA immunization were 100- and 1,000-fold higher via the i.m. or i.d. route, respectively. However, despite the generation of high-avidity Ab in DNA-immunized mice, germinal centers could not be detected in either DNA- or protein-immunized mice. Examination of the IgG subclass response showed that IgG2a was induced by i.m. DNA immunization, coinciding with elevated interferon gamma production, whereas a dominant and elevated IgG1 response, coinciding with detectable interleukin 4 production, was generated after i.d. immunization with DNA or soluble OVA and hen egg lysozyme but not human Ig protein. As expected, cytotoxic T cell (CTL) responses could be detected only after DNA immunization. I.d. immunization produced the strongest CTL responses early (2 weeks) but was similar to i.m. later. Therefore, DNA immunization can differ from protein immunization by its ability to induce rapid CTL responses and higher avidity Ab, both of which are advantageous for vaccination.
Figures
References
-
- Wolff J A, Williams P, Acsadi G, Jiao S, Jani A, Chong W. Biotechniques. 1991;11:474–485. - PubMed
-
- Tang D C, De Vit M, Johnston S A. Nature (London) 1992;356:152–154. - PubMed
-
- Yankauckas M A, Morrow J E, Parker S E, Abai A, Rhodes G H, Dwarki V J, Gromkowski S H. DNA Cell Biol. 1993;12:771–776. - PubMed
-
- Donnelly J J, Ulmer J B, Liu M A. Life Sci. 1997;60:163–172. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

