In vitro-generated neural precursors participate in mammalian brain development
- PMID: 9405695
- PMCID: PMC25119
- DOI: 10.1073/pnas.94.26.14809
In vitro-generated neural precursors participate in mammalian brain development
Abstract
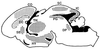
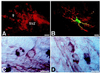
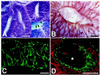
During embryogenesis, pluripotent stem cells segregate into daughter lineages of progressively restricted developmental potential. In vitro, this process has been mimicked by the controlled differentiation of embryonic stem cells into neural precursors. To explore the developmental potential of these cell-culture-derived precursors in vivo, we have implanted them into the ventricles of embryonic rats. The transplanted cells formed intraventricular neuroepithelial structures and migrated in large numbers into the brain tissue. Embryonic-stem-cell-derived neurons, astrocytes, and oligodendrocytes incorporated into telencephalic, diencephalic, and mesencephalic regions and assumed phenotypes indistinguishable from neighboring host cells. These observations indicate that entirely in vitro-generated neural precursors are able to respond to environmental signals guiding cell migration and differentiation and have the potential to reconstitute neuronal and glial lineages in the central nervous system.
Figures


References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

