Targeted gene deletion of heme oxygenase 2 reveals neural role for carbon monoxide
- PMID: 9405702
- PMCID: PMC25126
- DOI: 10.1073/pnas.94.26.14848
Targeted gene deletion of heme oxygenase 2 reveals neural role for carbon monoxide
Abstract
Neuronal nitric oxide synthase (nNOS) generates NO in neurons, and heme-oxygenase-2 (HO-2) synthesizes carbon monoxide (CO). We have evaluated the roles of NO and CO in intestinal neurotransmission using mice with targeted deletions of nNOS or HO-2. Immunohistochemical analysis demonstrated colocalization of nNOS and HO-2 in myenteric ganglia. Nonadrenergic noncholinergic relaxation and cyclic guanosine 3',5' monophosphate elevations evoked by electrical field stimulation were diminished markedly in both nNOSDelta/Delta and HO-2(Delta)/Delta mice. In wild-type mice, NOS inhibitors and HO inhibitors partially inhibited nonadrenergic noncholinergic relaxation. In nNOSDelta/Delta animals, NOS inhibitors selectively lost their efficacy, and HO inhibitors were inactive in HO-2(Delta)/Delta animals.
Figures

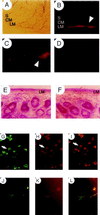

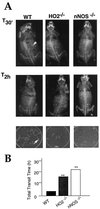
References
-
- Maines M D. Annu Rev Pharmacol Toxicol. 1997;37:517–554. - PubMed
-
- Jaffrey S R, Snyder S H. Annu Rev Cell Dev Biol. 1995;11:417–440. - PubMed
-
- Bult H, Boeckxstaens G E, Pelckmans P A, Jordaens F H, Van Maercke Y M, Herman A G. Nature (London) 1990;345:346–347. - PubMed
-
- Verma A, Hirsch D J, Glatt C E, Ronnett G V, Snyder S H. Science. 1993;259:381–384. - PubMed
-
- Bredt D S, Hwang P M, Snyder S H. Nature (London) 1990;347:768–770. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

