The phosphoprotein DARPP-32 mediates cAMP-dependent potentiation of striatal N-methyl-D-aspartate responses
- PMID: 9405704
- PMCID: PMC25128
- DOI: 10.1073/pnas.94.26.14859
The phosphoprotein DARPP-32 mediates cAMP-dependent potentiation of striatal N-methyl-D-aspartate responses
Abstract
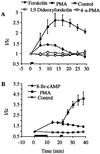
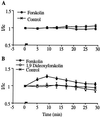
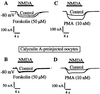
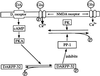
The signal transduction pathway underlying the cAMP-dependent modulation of rat striatal N-methyl-D-aspartate (NMDA) responses was investigated by using the two-electrode voltage-clamp technique. In oocytes injected with rat striatal poly(A)+ mRNA, activation of cAMP-dependent protein kinase (PKA) by forskolin potentiated NMDA responses. Inhibition of protein phosphatase 1 (PP1) and/or protein phosphatase 2A (PP2A) by the specific inhibitor calyculin A occluded the PKA-mediated potentiation of striatal NMDA responses, suggesting that the PKA effect was mediated by inhibition of a protein phosphatase. Coinjection of oocytes with striatal mRNA and antisense oligodeoxynucleotides directed against the protein phosphatase inhibitor DARPP-32 dramatically reduced the PKA enhancement of NMDA responses. NMDA responses recorded from oocytes injected with rat hippocampal poly(A)+ mRNA were not affected by stimulation of PKA. When oocytes were coinjected with rat hippocampal poly(A)+ mRNA plus complementary RNA coding for DARPP-32, NMDA responses were potentiated after stimulation of PKA. The results provide evidence that DARPP-32, which is enriched in the striatum, may participate in the signaling between the two major afferent striatal pathways, the glutamatergic and the dopaminergic projections, by the cAMP-dependent regulation of striatal NMDA currents.
Figures


References
-
- Voorn P, Jorritsma-Byham B, Van Dijk B, Buijs R M. J Comp Neurol. 1986;251:84–99. - PubMed
-
- Doucet G, Descarries L, Garcia S. Neuroscience. 1986;19:427–445. - PubMed
-
- McGeer P L, McGeer E G, Scherer U, Singh K. Brain Res. 1977;128:369–373. - PubMed
-
- Fonnum F, Storm-Mathisen J, Divac I. Neuroscience. 1981;6:863–873. - PubMed
-
- Dube L, Smith A D, Bolam J P. J Comp Neurol. 1988;267:455–471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

