5'-Capping enzymes are targeted to pre-mRNA by binding to the phosphorylated carboxy-terminal domain of RNA polymerase II
- PMID: 9407024
- PMCID: PMC316822
- DOI: 10.1101/gad.11.24.3306
5'-Capping enzymes are targeted to pre-mRNA by binding to the phosphorylated carboxy-terminal domain of RNA polymerase II
Abstract
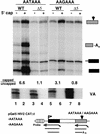
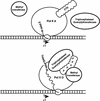
We have investigated the role of the RNA Polymerase II (Pol II) carboxy-terminal domain (CTD) in mRNA 5' capping. Transcripts made in vivo by Pol II with a truncated CTD had a lower proportion of capped 5' ends than those made by Pol II with a full-length CTD. In addition, the enzymes responsible for cap synthesis, RNA guanylyltransferase, and RNA (guanine-7)-methyltransferase bound directly to the phosphorylated, but not to the nonphosphorylated, form of the CTD in vitro. These results suggest that: (1) Pol II-specific capping of nascent transcripts in vivo is enhanced by recruitment of the capping enzymes to the CTD and (2) capping is co-ordinated with CTD phosphorylation.
Figures





Comment in
-
Transcription units as RNA processing units.Genes Dev. 1997 Dec 15;11(24):3279-85. doi: 10.1101/gad.11.24.3279. Genes Dev. 1997. PMID: 9407022 Review. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
