Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast
- PMID: 9407035
- PMCID: PMC316814
- DOI: 10.1101/gad.11.24.3432
Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast
Abstract
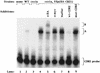
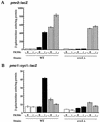
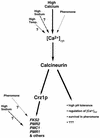
Calcineurin is a conserved Ca2+/calmodulin-dependent protein phosphatase that plays a critical role in Ca2+ signaling. We describe new components of a calcineurin-mediated response in yeast, the Ca2+-induced transcriptional activation of FKS2, which encodes a beta-1,3 glucan synthase. A 24-bp region of the FKS2 promoter was defined as sufficient to confer calcineurin-dependent transcriptional induction on a minimal promoter in response to Ca2+ and was named CDRE (for calcineurin-dependent response element). The product of CRZ1 (YNL027w) was identified as an activator of CDRE-driven transcription. Crz1p contains zinc finger motifs and binds specifically to the CDRE. Genetic analysis revealed that crz1Delta mutant cells exhibit several phenotypes similar to those of calcineurin mutants and that overexpression of CRZ1 in calcineurin mutants suppressed these phenotypes. These results suggest that Crz1p functions downstream of calcineurin to effect multiple calcineurin-dependent responses. Moreover, the calcineurin-dependent transcriptional induction of FKS2 in response to Ca2+, alpha-factor, and Na+ was found to require CRZ1. In addition, we found that the calcineurin-dependent transcriptional regulation of PMR2 and PMC1 required CRZ1. However, transcription of PMR2 and PMC1 was activated by only a subset of the treatments that activated FKS2 transcription. Thus, in response to multiple signals, calcineurin acts through the Crz1p transcription factor to differentially regulate the expression of several target genes in yeast.
Figures






References
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current protocols in molecular biology. 1 and 2. New York, NY: John Wiley and Sons; 1987.
-
- Clapham DE. Calcium signaling. Cell. 1995;80:259–268. - PubMed
-
- Clipstone NA, Crabtree GR. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992;357:695–697. - PubMed
-
- Cox JS, Walter P. A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell. 1996;87:391–404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
