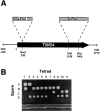
The Tim54p-Tim22p complex mediates insertion of proteins into the mitochondrial inner membrane
- PMID: 9412462
- PMCID: PMC2132641
- DOI: 10.1083/jcb.139.7.1663
The Tim54p-Tim22p complex mediates insertion of proteins into the mitochondrial inner membrane
Abstract
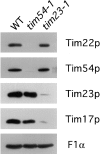
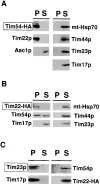
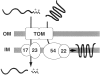
We have identified a new protein, Tim54p, located in the yeast mitochondrial inner membrane. Tim54p is an essential import component, required for the insertion of at least two polytopic proteins into the inner membrane, but not for the translocation of precursors into the matrix. Several observations suggest that Tim54p and Tim22p are part of a protein complex in the inner membrane distinct from the previously characterized Tim23p-Tim17p complex. First, multiple copies of the TIM22 gene, but not TIM23 or TIM17, suppress the growth defect of a tim54-1 temperature-sensitive mutant. Second, Tim22p can be coprecipitated with Tim54p from detergent-solubilized mitochondria, but Tim54p and Tim22p do not interact with either Tim23p or Tim17p. Finally, the tim54-1 mutation destabilizes the Tim22 protein, but not Tim23p or Tim17p. Our results support the idea that the mitochondrial inner membrane carries two independent import complexes: one required for the translocation of proteins across the inner membrane (Tim23p-Tim17p), and the other required for the insertion of proteins into the inner membrane (Tim54p-Tim22p).
Figures



Similar articles
-
Tim18p is a new component of the Tim54p-Tim22p translocon in the mitochondrial inner membrane.Mol Biol Cell. 2000 Jan;11(1):103-16. doi: 10.1091/mbc.11.1.103. Mol Biol Cell. 2000. PMID: 10637294 Free PMC article.
-
Tim18p, a new subunit of the TIM22 complex that mediates insertion of imported proteins into the yeast mitochondrial inner membrane.Mol Cell Biol. 2000 Feb;20(4):1187-93. doi: 10.1128/MCB.20.4.1187-1193.2000. Mol Cell Biol. 2000. PMID: 10648604 Free PMC article.
-
Two intermembrane space TIM complexes interact with different domains of Tim23p during its import into mitochondria.J Cell Biol. 2000 Sep 18;150(6):1271-82. doi: 10.1083/jcb.150.6.1271. J Cell Biol. 2000. PMID: 10995434 Free PMC article.
-
Protein import into and across the mitochondrial inner membrane: role of the TIM23 and TIM22 translocons.Biochim Biophys Acta. 2002 Sep 2;1592(1):25-34. doi: 10.1016/s0167-4889(02)00261-6. Biochim Biophys Acta. 2002. PMID: 12191765 Review.
-
The preprotein translocase of the mitochondrial inner membrane: function and evolution.J Mol Biol. 1999 Feb 12;286(1):105-20. doi: 10.1006/jmbi.1998.2455. J Mol Biol. 1999. PMID: 9931253 Review.
Cited by
-
Defective mitochondrial disulfide relay system, altered mitochondrial morphology and function in Huntington's disease.Hum Mol Genet. 2013 Mar 1;22(5):989-1004. doi: 10.1093/hmg/dds503. Epub 2012 Nov 29. Hum Mol Genet. 2013. PMID: 23197653 Free PMC article.
-
Protein translocase of mitochondrial inner membrane in Trypanosoma brucei.J Biol Chem. 2012 Apr 27;287(18):14480-93. doi: 10.1074/jbc.M111.322925. Epub 2012 Mar 9. J Biol Chem. 2012. PMID: 22408251 Free PMC article.
-
MCC and PSC, the putative protein import channels of mitochondria.J Bioenerg Biomembr. 2000 Feb;32(1):47-54. doi: 10.1023/a:1005560328334. J Bioenerg Biomembr. 2000. PMID: 11768761 Review.
-
Essential role of Mia40 in import and assembly of mitochondrial intermembrane space proteins.EMBO J. 2004 Oct 1;23(19):3735-46. doi: 10.1038/sj.emboj.7600389. Epub 2004 Sep 9. EMBO J. 2004. PMID: 15359280 Free PMC article.
-
The assembly pathway of the mitochondrial carrier translocase involves four preprotein translocases.Mol Cell Biol. 2008 Jul;28(13):4251-60. doi: 10.1128/MCB.02216-07. Epub 2008 May 5. Mol Cell Biol. 2008. PMID: 18458057 Free PMC article.
References
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Armstrong KA, Som T, Volkert FC, Rose A, Broach JR. Propagation and expression of genes in yeast using 2-micron circle vectors. Biotechnology. 1989;13:165–192. - PubMed
-
- Bachhawat AK, Suhan J, Jones EW. The yeast homolog of H<β>58, a mouse gene essential for embryogenesis, performs a role in the delivery of proteins to the vacuole. Genes Dev. 1994;8:1379–1387. - PubMed
-
- Bai C, Elledge SJ. Gene identification using the yeast two-hybrid system. Methods Enzymol. 1996;273:331–347. - PubMed
-
- Bauer M, Sirrenberg C, Neupert W, Brunner M. Role of Tim23 as voltage sensor and presequence receptor in protein import into mitochondria. Cell. 1996;87:33–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

