Ultrastructural organization of bovine chromaffin cell cortex-analysis by cryofixation and morphometry of aspects pertinent to exocytosis
- PMID: 9412466
- PMCID: PMC2132648
- DOI: 10.1083/jcb.139.7.1709
Ultrastructural organization of bovine chromaffin cell cortex-analysis by cryofixation and morphometry of aspects pertinent to exocytosis
Erratum in
- J Cell Biol 1998 Feb 23;140(4):973
Abstract
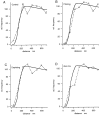
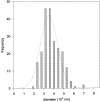
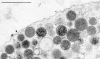
We have analyzed ultrathin sections from isolated bovine chromaffin cells grown on plastic support, after fast freezing, by quantitative electron microscopy. We determined the size and intracellular distribution of dense core vesicles (DVs or chromaffin granules) and of clear vesicles (CVs). The average diameter of DVs is 356 nm, and that of CVs varies between 35-195 nm (average 90 nm). DVs appear randomly packed inside cells. When the distance of the center of DVs to the cell membrane (CM) is analyzed, DV density is found to decrease as the CM is approached. According to Monte Carlo simulations performed on the basis of the measured size distribution of DVs, this decay can be assigned to a "wall effect." Any cortical barrier, regardless of its function, seems to not impose a restriction to a random cortical DV packing pattern. The number of DVs closely approaching the CM (docked DVs) is estimated to be between 364 and 629 (average 496), i.e., 0.45 to 0.78 DVs/micron2 CM. Deprivation of Ca2+, priming by increasing [Ca2+]i, or depolarization by high [K+]e for 10 s (the effect of which was controlled electrophysiologically and predicted to change the number of readily releasable granules [RRGs]) does not significantly change the number of peripheral DVs. The reason may be that (a) structural docking implies only in part functional docking (capability of immediate release), and (b) exocytosis is rapidly followed by endocytosis and replenishment of the pool of docked DVs. Whereas the potential contribution of DVs to CM area increase by immediate release can be estimated at 19-33%, that of CVs is expected to be in the range of 5.6-8.0%.
Figures




References
-
- Augustine GJ, Burns ME, DeBello WM, Pettit DL, Schweizer FE. Exocytosis: proteins and perturbations. Annu Rev Pharmacol Toxicol. 1996;36:659–701. - PubMed
-
- Aunis D, Bader MF. The cytoskeleton as a barrier to exocytosis in secretory cells. J Exp Biol. 1988;139:253–266. - PubMed
-
- Bittner MA, Holz RW. Kinetic analysis of secretion from permeabilized adrenal chromaffin cells reveals distinct components. J Biol Chem. 1992;267:16219–16225. - PubMed
-
- Bommert K, Charlton MP, DeBello WM, Chin GJ, Betz H, Augustine GJ. Inhibition of neurotransmitter release by C2-domain peptides implicates synaptotagmin in exocytosis. Nature. 1997;363:163–165. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

