Actin-binding verprolin is a polarity development protein required for the morphogenesis and function of the yeast actin cytoskeleton
- PMID: 9412475
- PMCID: PMC2132640
- DOI: 10.1083/jcb.139.7.1821
Actin-binding verprolin is a polarity development protein required for the morphogenesis and function of the yeast actin cytoskeleton
Abstract
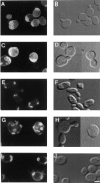
Yeast verprolin, encoded by VRP1, is implicated in cell growth, cytoskeletal organization, endocytosis and mitochondrial protein distribution and function. We show that verprolin is also required for bipolar bud-site selection. Previously we reported that additional actin suppresses the temperature-dependent growth defect caused by a mutation in VRP1. Here we show that additional actin suppresses all known defects caused by vrp1-1 and conclude that the defects relate to an abnormal cytoskeleton. Using the two-hybrid system, we show that verprolin binds actin. An actin-binding domain maps to the LKKAET hexapeptide located in the first 70 amino acids. A similar hexapeptide in other acting-binding proteins was previously shown to be necessary for actin-binding activity. The entire 70- amino acid motif is conserved in novel higher eukaryotic proteins that we predict to be actin-binding, and also in the actin-binding proteins, WASP and N-WASP. Verprolin-GFP in live cells has a cell cycle-dependent distribution similar to the actin cortical cytoskeleton. In fixed cells hemagglutinin-tagged Vrp1p often co-localizes with actin in cortical patches. However, disassembly of the actin cytoskeleton using Latrunculin-A does not alter verprolin's location, indicating that verprolin establishes and maintains its location independent of the actin cytoskeleton. Verprolin is a new member of the actin-binding protein family that serves as a polarity development protein, perhaps by anchoring actin. We speculate that the effects of verprolin upon the actin cytoskeleton might influence mitochondrial protein sorting/function via mRNA distribution.
Figures







Similar articles
-
Verprolin function in endocytosis and actin organization. Roles of the Las17p (yeast WASP)-binding domain and a novel C-terminal actin-binding domain.FEBS J. 2007 Aug;274(16):4103-25. doi: 10.1111/j.1742-4658.2007.05936.x. Epub 2007 Jul 16. FEBS J. 2007. PMID: 17635585
-
The human WASP-interacting protein, WIP, activates the cell polarity pathway in yeast.J Biol Chem. 1999 Jun 11;274(24):17103-8. doi: 10.1074/jbc.274.24.17103. J Biol Chem. 1999. PMID: 10358064
-
The Src homology domain 3 (SH3) of a yeast type I myosin, Myo5p, binds to verprolin and is required for targeting to sites of actin polarization.J Cell Biol. 1998 Jun 15;141(6):1357-70. doi: 10.1083/jcb.141.6.1357. J Cell Biol. 1998. PMID: 9628892 Free PMC article.
-
Verprolin: a cool set of actin-binding sites and some very HOT prolines.IUBMB Life. 2009 Jul;61(7):707-12. doi: 10.1002/iub.195. IUBMB Life. 2009. PMID: 19507265 Review.
-
The verprolin family of proteins: regulators of cell morphogenesis and endocytosis.FEBS Lett. 2005 Oct 10;579(24):5253-9. doi: 10.1016/j.febslet.2005.08.053. FEBS Lett. 2005. PMID: 16182290 Review.
Cited by
-
Phospholipid flippases Lem3p-Dnf1p and Lem3p-Dnf2p are involved in the sorting of the tryptophan permease Tat2p in yeast.J Biol Chem. 2013 Feb 1;288(5):3594-608. doi: 10.1074/jbc.M112.416263. Epub 2012 Dec 18. J Biol Chem. 2013. PMID: 23250744 Free PMC article.
-
Scar/WAVE-1, a Wiskott-Aldrich syndrome protein, assembles an actin-associated multi-kinase scaffold.EMBO J. 2000 Sep 1;19(17):4589-600. doi: 10.1093/emboj/19.17.4589. EMBO J. 2000. PMID: 10970852 Free PMC article.
-
MIM-B, a putative metastasis suppressor protein, binds to actin and to protein tyrosine phosphatase delta.Biochem J. 2003 Apr 15;371(Pt 2):463-71. doi: 10.1042/BJ20021962. Biochem J. 2003. PMID: 12570871 Free PMC article.
-
Imaging the Actin Cytoskeleton in Live Budding Yeast Cells.Methods Mol Biol. 2022;2364:53-80. doi: 10.1007/978-1-0716-1661-1_3. Methods Mol Biol. 2022. PMID: 34542848 Free PMC article.
-
MoVrp1, a putative verprolin protein, is required for asexual development and infection in the rice blast fungus Magnaporthe oryzae.Sci Rep. 2017 Jan 24;7:41148. doi: 10.1038/srep41148. Sci Rep. 2017. PMID: 28117435 Free PMC article.
References
-
- Adams, A.E., and J.R. Pringle. 1991. Staining of actin with fluorochrome-conjugated phalloidin. In Methods Enzymol. Guide to Yeast Genetics and Molecular Biology; Vol. 194. C. Guthrie and G.R. Fink, editors. Academic Press Inc., San Diego, CA. 729–731. - PubMed
-
- Amberg DC, Basart E, Botstein D. Defining protein interactions with yeast actin in vivo. . Nat Struct Biol. 1995;2:28–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

