Effects of the metabotropic glutamate receptor antagonist MCPG on phosphoinositide turnover and synaptic plasticity in visual cortex
- PMID: 9412480
- PMCID: PMC6793393
- DOI: 10.1523/JNEUROSCI.18-01-00001.1998
Effects of the metabotropic glutamate receptor antagonist MCPG on phosphoinositide turnover and synaptic plasticity in visual cortex
Abstract
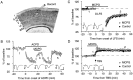
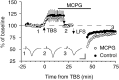
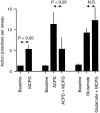
The neurotransmitter glutamate, in addition to activating ligand-gated ion channels, also stimulates phosphoinositide (PI) hydrolysis in neurons by activating a group of G-protein-coupled metabotropic glutamate receptors (mGluRs). A role for mGluRs in synaptic plasticity originally was hypothesized based on the observation that the developmental decline in glutamate-stimulated PI turnover is well correlated with the decline in experience-dependent synaptic plasticity in visual cortex. Over the past few years, the compound alpha-methyl-4-carboxyphenylglycine (MCPG) has been widely used to test the role of PI-coupled mGluRs in a number of types of synaptic plasticity, including long-term potentiation (LTP), long-term depression (LTD), ocular dominance plasticity in visual cortex, and the neural plasticity underlying learning and memory. The conclusions of most of these studies were based on the assumption that MCPG blocks the actions of glutamate at PI-coupled mGluRs in the cerebral cortex. Here we show that this assumption is not valid in visual cortex. Although MCPG does antagonize the actions of the synthetic mGluR agonist 1S, 3R-aminocyclopentane-1,3-dicarboxylic acid, it fails to block PI turnover and changes in spike adaptation stimulated by glutamate, the endogenous mGluR ligand. In addition, we find that MCPG fails to block the NMDA receptor-dependent forms of LTP, LTD, and depotentiation in visual cortex.
Figures




References
-
- Abe T, Sugihara H, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J Biol Chem. 1992;267:13361–13368. - PubMed
-
- Aizenman CD, Kirkwood A, Bear MF. Current source density analysis of evoked responses in visual cortex in vitro: implications for the regulation of long-term potentiation. Cereb Cortex. 1996;16:751–758. - PubMed
-
- Bashir ZI, Bortolotto ZA, Davies CH, Berretta N, Irving AJ, Seal AJ, Henley JM, Jane DS, Watkins JC, Collingridge GL. Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature. 1993;363:347–350. - PubMed
-
- Bashir ZI, Collingridge GL. An investigation of depotentiation of long-term potentiation in the CA1 region of the hippocampus. Exp Brain Res. 1994;100:437–443. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
