A focal zone of thalamic plasticity
- PMID: 9412530
- PMCID: PMC6793409
- DOI: 10.1523/JNEUROSCI.18-01-00548.1998
A focal zone of thalamic plasticity
Abstract
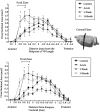
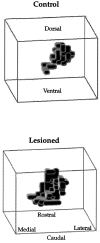
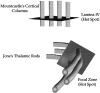
In this study, sensory maps in the thalamus were investigated by examining their volume and shape. We determined the forelimb representation in adult rats after the removal of hindlimb input by nucleus gracilis lesions. Three-dimensional reconstructions of thalamic sensory maps were obtained from a grid of electrode penetrations. We found that the volume of the shoulder sensory map contracted >50% at an acute time interval (n = 6), followed by a robust volumetric sensory map expansion of 25% at 1 week (n = 8) and 1 month (n = 8) after lesion relative to controls (n = 8). The topology of the volumetric increase was scrutinized by slicing functional maps in the coronal, sagittal, and horizontal planes. The equivalence of such slices from each animal was established by virtue of their distance from either a functional or neuroanatomical landmark. Surprisingly, all of the volumetric increase unequivocally occurred in a circumscribed coronal slice 300 micron thick. This focal zone was located toward the rostral pole of the thalamic tactile relay, the ventroposterolateral nucleus. Analysis in the sagittal plane revealed that, unexpectedly, the shoulder map volume expanded by superimposing its representation on that of the forepaw, via an advancement of the shoulder representation by 0.6 mm medially. We propose a "hot spot" hypothesis in which focal zones of plasticity may not be specific to the thalamus but may have manifestations elsewhere in the nervous system, such as the cerebral cortex or dorsal column nuclei.
Figures








Similar articles
-
Cortical involvement in the induction, but not expression, of thalamic plasticity.J Neurosci. 1999 Oct 1;19(19):8623-9. doi: 10.1523/JNEUROSCI.19-19-08623.1999. J Neurosci. 1999. PMID: 10493762 Free PMC article.
-
Large-scale reorganization in the somatosensory cortex and thalamus after sensory loss in macaque monkeys.J Neurosci. 2008 Oct 22;28(43):11042-60. doi: 10.1523/JNEUROSCI.2334-08.2008. J Neurosci. 2008. PMID: 18945912 Free PMC article.
-
Tactile impoverishment and sensorimotor restriction deteriorate the forepaw cutaneous map in the primary somatosensory cortex of adult rats.Exp Brain Res. 1999 Dec;129(4):518-31. doi: 10.1007/s002210050922. Exp Brain Res. 1999. PMID: 10638426
-
Intrathalamic sensory connections mediated by the thalamic reticular nucleus.Cell Mol Life Sci. 1999 Nov 15;56(7-8):683-700. doi: 10.1007/s000180050462. Cell Mol Life Sci. 1999. PMID: 11212315 Free PMC article. Review.
-
Network level properties of short-term plasticity in the somatosensory system.Prog Brain Res. 2000;128:161-72. doi: 10.1016/S0079-6123(00)28014-X. Prog Brain Res. 2000. PMID: 11105676 Review. No abstract available.
Cited by
-
Cortical involvement in the induction, but not expression, of thalamic plasticity.J Neurosci. 1999 Oct 1;19(19):8623-9. doi: 10.1523/JNEUROSCI.19-19-08623.1999. J Neurosci. 1999. PMID: 10493762 Free PMC article.
-
Progressive transneuronal changes in the brainstem and thalamus after long-term dorsal rhizotomies in adult macaque monkeys.J Neurosci. 2000 May 15;20(10):3884-99. doi: 10.1523/JNEUROSCI.20-10-03884.2000. J Neurosci. 2000. PMID: 10804228 Free PMC article.
-
Delayed reorganization of the shoulder representation in forepaw barrel subfield (FBS) in first somatosensory cortex (SI) following forelimb deafferentation in adult rats.Exp Brain Res. 2003 Nov;153(1):100-12. doi: 10.1007/s00221-003-1625-z. Epub 2003 Aug 29. Exp Brain Res. 2003. PMID: 12955377
References
-
- Alloway KD, Aaron GB. Adaptive changes in the somatotopic properties of individual thalamic neurons immediately following microlesions in connected regions of the nucleus cuneatus. Synapse. 1996;22:1–14. - PubMed
-
- Basbaum AI, Wall PD. Chronic changes in the response of cells in adult cat dorsal horn following partial deafferentation: the appearance of responding cells in a previously non-responsive region. Brain Res. 1976;116:181–204. - PubMed
-
- Calford MB, Tweedale R. Interhemispheric transfer of plasticity in the rat cerebral cortex. Science. 1990;249:805–807. - PubMed
-
- Dado RJ, Katter JT, Giesler GJJ. Spinothalamic and spinohypothalamic tract neurons in the cervical enlargement of rats. II. Responses to innocuous and noxious mechanical mechanical and thermal stimuli. J Neurophysiol. 1994;71:981–1002. - PubMed
-
- Devor M, Wall PD. Reorganization of spinal cord sensory map after peripheral nerve injury. Nature. 1978;275:75–76. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
