Voltage-dependent membrane displacements measured by atomic force microscopy
- PMID: 9417135
- PMCID: PMC1887771
- DOI: 10.1085/jgp.111.1.65
Voltage-dependent membrane displacements measured by atomic force microscopy
Abstract
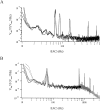
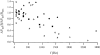
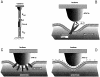
Cells use polar molecules in the membrane to sense changes in the transmembrane potential. The opening of voltage-gated ion channels and membrane bending due to the inverse flexoelectric effect are two examples of such electromechanical coupling. We have looked for membrane motions in an electric field using atomic (or scanning) force microscopy (AFM) with the intent of studying voltage-dependent conformational changes of ion channels. Voltage-clamped HEK293 cells were either untransfected controls or transfected with Shaker K+ channels. Using a +/- 10-mV peak-peak AC carrier stimulus, untransfected cells moved 0.5-15 nm normal to the plane of the membrane. These movements tracked the voltage at frequencies >1 kHz with a phase lead of 60-120 degrees, as expected of a displacement current. The movement was outward with depolarization, but the holding potential only weakly influenced the amplitude of the movement. In contrast, cells transfected with a noninactivating mutant of Shaker K+channels showed similar movements, but these were sensitive to the holding potential; decreasing with depolarization between -80 and 0 mV. Searching for artifactual origins of these movements, we used open or sealed pipettes and AFM cantilever placements just above the cells. These results were negative, suggesting that the observed movements were produced by the cell membrane rather than by movement of the patch pipette, or by acoustic or electrical interactions of the membrane with the AFM tip. In control cells, the electrical motor may arise from the flexoelectric effect, where changes in potential induce changes in curvature. In transfected cells, it appears that channel-specific movements also occurred. These experiments demonstrate that the AFM may be able to exploit voltage-dependent movements as a source of contrast for imaging membrane components. The electrically induced motility will cause twitching during action potentials, and may have physiological consequences.
Figures



Similar articles
-
Electromechanical coupling in the membranes of Shaker-transfected HEK cells.Proc Natl Acad Sci U S A. 2009 Apr 21;106(16):6626-31. doi: 10.1073/pnas.0808045106. Epub 2009 Apr 6. Proc Natl Acad Sci U S A. 2009. PMID: 19366664 Free PMC article.
-
Voltage-insensitive gating after charge-neutralizing mutations in the S4 segment of Shaker channels.J Gen Physiol. 1999 Jan;113(1):139-51. doi: 10.1085/jgp.113.1.139. J Gen Physiol. 1999. PMID: 9874694 Free PMC article.
-
Kvbeta1.2 subunit coexpression in HEK293 cells confers O2 sensitivity to kv4.2 but not to Shaker channels.J Gen Physiol. 1999 Jun;113(6):897-907. doi: 10.1085/jgp.113.6.897. J Gen Physiol. 1999. PMID: 10352037 Free PMC article.
-
Structure and function of potassium channels in plants: some inferences about the molecular origin of inward rectification in KAT1 channels (Review).Mol Membr Biol. 2003 Jan-Mar;20(1):19-25. doi: 10.1080/0968768021000057371. Mol Membr Biol. 2003. PMID: 12745922 Review.
-
Shedding light on voltage-dependent gating.J Gen Physiol. 1998 Oct;112(4):373-6. doi: 10.1085/jgp.112.4.373. J Gen Physiol. 1998. PMID: 9758857 Free PMC article. Review. No abstract available.
Cited by
-
Electromechanical coupling in the membranes of Shaker-transfected HEK cells.Proc Natl Acad Sci U S A. 2009 Apr 21;106(16):6626-31. doi: 10.1073/pnas.0808045106. Epub 2009 Apr 6. Proc Natl Acad Sci U S A. 2009. PMID: 19366664 Free PMC article.
-
Optical Electrophysiology: Toward the Goal of Label-Free Voltage Imaging.J Am Chem Soc. 2021 Jul 21;143(28):10482-10499. doi: 10.1021/jacs.1c02960. Epub 2021 Jun 30. J Am Chem Soc. 2021. PMID: 34191488 Free PMC article.
-
Nanotechnology in Auditory Research: Membrane Electromechanics in Hearing.Methods Mol Biol. 2016;1427:349-62. doi: 10.1007/978-1-4939-3615-1_20. Methods Mol Biol. 2016. PMID: 27259937 Free PMC article. Review.
-
Imaging single photons and intrinsic optical signals for studies of vesicular and non-vesicular ATP release from axons.Front Neuroanat. 2011 Jun 6;5:32. doi: 10.3389/fnana.2011.00032. eCollection 2011. Front Neuroanat. 2011. PMID: 21852965 Free PMC article.
-
Membrane stretch affects gating modes of a skeletal muscle sodium channel.Biophys J. 1999 Aug;77(2):758-74. doi: 10.1016/S0006-3495(99)76930-4. Biophys J. 1999. PMID: 10423424 Free PMC article.
References
-
- Ashmore, J.F. 1989. Transducer motor coupling in cochlear outer hair cells. In Cochlear Mechanisms, Structure, Function and Models. J.P. Wilson and D.T. Kemp, editors. Plenum Publishing Corp., New York. 109–114.
-
- Barber K, Enam SA, Bodovitz S, Falduto M, Frail D, Klein WL. Particulate forms of APP in the extracellular milieu of cultured cells. Exp Neurol. 1995;132:42–53. - PubMed
-
- Bezanilla F, Perozo E, Papazian DM, Stefani E. Molecular basis of gating charge immobilization in Shaker potassium channels. Science. 1991;254:679–683. - PubMed
-
- Bezanilla F, Stefani E. Voltage-dependent gating of ionic channels. Annu Rev Biophys Biomol Struct. 1994;23:819–846. - PubMed
-
- Binnig G, Quate CF, Gerber C. Atomic force microscope. Physical Review Letters. 1986;56:930–933. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

