Slow inactivation does not affect movement of the fast inactivation gate in voltage-gated Na+ channels
- PMID: 9417137
- PMCID: PMC1887763
- DOI: 10.1085/jgp.111.1.83
Slow inactivation does not affect movement of the fast inactivation gate in voltage-gated Na+ channels
Abstract
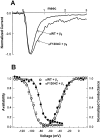
Voltage-gated Na+ channels exhibit two forms of inactivation, one form (fast inactivation) takes effect on the order of milliseconds and the other (slow inactivation) on the order of seconds to minutes. While previous studies have suggested that fast and slow inactivation are structurally independent gating processes, little is known about the relationship between the two. In this study, we probed this relationship by examining the effects of slow inactivation on a conformational marker for fast inactivation, the accessibility of a site on the Na+ channel III-IV linker that is believed to form a part of the fast inactivation particle. When cysteine was substituted for phenylalanine at position 1304 in the rat skeletal muscle sodium channel (microl), application of [2-(trimethylammonium)ethyl]methanethiosulfonate (MTS-ET) to the cytoplasmic face of inside-out patches from Xenopus oocytes injected with F1304C RNA dramatically disrupted fast inactivation and displayed voltage-dependent reaction kinetics that closely paralleled the steady state availability (hinfinity) curve. Based on this observation, the accessibility of cys1304 was used as a conformational marker to probe the position of the fast inactivation gate during the development of and the recovery from slow inactivation. We found that burial of cys1304 is not altered by the onset of slow inactivation, and that recovery of accessibility of cys1304 is not slowed after long (2-10 s) depolarizations. These results suggest that (a) fast and slow inactivation are structurally distinct processes that are not tightly coupled, (b) fast and slow inactivation are not mutually exclusive processes (i.e., sodium channels may be fast- and slow-inactivated simultaneously), and (c) after long depolarizations, recovery from fast inactivation precedes recovery from slow inactivation.
Figures





Similar articles
-
The position of the fast-inactivation gate during lidocaine block of voltage-gated Na+ channels.J Gen Physiol. 1999 Jan;113(1):7-16. doi: 10.1085/jgp.113.1.7. J Gen Physiol. 1999. PMID: 9874684 Free PMC article.
-
External pore residue mediates slow inactivation in mu 1 rat skeletal muscle sodium channels.J Physiol. 1996 Jul 15;494 ( Pt 2)(Pt 2):431-42. doi: 10.1113/jphysiol.1996.sp021503. J Physiol. 1996. PMID: 8842002 Free PMC article.
-
Slow inactivation does not block the aqueous accessibility to the outer pore of voltage-gated Na channels.J Gen Physiol. 2002 Oct;120(4):509-16. doi: 10.1085/jgp.20028672. J Gen Physiol. 2002. PMID: 12356853 Free PMC article.
-
Comparison of slow inactivation in human heart and rat skeletal muscle Na+ channel chimaeras.J Physiol. 1999 Feb 15;515 ( Pt 1)(Pt 1):61-73. doi: 10.1111/j.1469-7793.1999.061ad.x. J Physiol. 1999. PMID: 9925878 Free PMC article.
-
Accessory subunits and sodium channel inactivation.Curr Opin Neurobiol. 1993 Jun;3(3):272-7. doi: 10.1016/0959-4388(93)90117-h. Curr Opin Neurobiol. 1993. PMID: 8396472 Review.
Cited by
-
Inhibition of Nav1.7 channels by methyl eugenol as a mechanism underlying its antinociceptive and anesthetic actions.Acta Pharmacol Sin. 2015 Jul;36(7):791-9. doi: 10.1038/aps.2015.26. Epub 2015 Jun 8. Acta Pharmacol Sin. 2015. PMID: 26051112 Free PMC article.
-
Slow sodium channel inactivation and use-dependent block modulated by the same domain IV S6 residue.J Membr Biol. 2005 Sep;207(2):107-17. doi: 10.1007/s00232-005-0805-0. J Membr Biol. 2005. PMID: 16477531
-
Sodium channelopathies of skeletal muscle result from gain or loss of function.Pflugers Arch. 2010 Jul;460(2):239-48. doi: 10.1007/s00424-010-0814-4. Epub 2010 Mar 17. Pflugers Arch. 2010. PMID: 20237798 Free PMC article. Review.
-
Functional characterization and cold sensitivity of T1313A, a new mutation of the skeletal muscle sodium channel causing paramyotonia congenita in humans.J Physiol. 2004 Feb 1;554(Pt 3):635-47. doi: 10.1113/jphysiol.2003.053082. Epub 2003 Nov 14. J Physiol. 2004. PMID: 14617673 Free PMC article.
-
Enhanced slow inactivation by V445M: a sodium channel mutation associated with myotonia.Biophys J. 1999 Feb;76(2):861-8. doi: 10.1016/S0006-3495(99)77249-8. Biophys J. 1999. PMID: 9929487 Free PMC article.
References
-
- Baukrowitz T, Yellen G. Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron. 1995;15:951–960. - PubMed

