Protease modulation of the activity of the epithelial sodium channel expressed in Xenopus oocytes
- PMID: 9417140
- PMCID: PMC1887769
- DOI: 10.1085/jgp.111.1.127
Protease modulation of the activity of the epithelial sodium channel expressed in Xenopus oocytes
Abstract
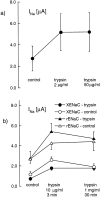
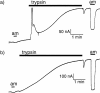
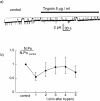
We have investigated the effect of extracellular proteases on the amiloride-sensitive Na+ current (INa) in Xenopus oocytes expressing the three subunits alpha, beta, and gamma of the rat or Xenopus epithelial Na+ channel (ENaC). Low concentrations of trypsin (2 microg/ml) induced a large increase of INa within a few minutes, an effect that was fully prevented by soybean trypsin inhibitor, but not by amiloride. A similar effect was observed with chymotrypsin, but not with kallikrein. The trypsin-induced increase of INa was observed with Xenopus and rat ENaC, and was very large (approximately 20-fold) with the channel obtained by coexpression of the alpha subunit of Xenopus ENaC with the beta and gamma subunits of rat ENaC. The effect of trypsin was selective for ENaC, as shown by the absence of effect on the current due to expression of the K+ channel ROMK2. The effect of trypsin was not prevented by intracellular injection of EGTA nor by pretreatment with GTP-gammaS, suggesting that this effect was not mediated by G proteins. Measurement of the channel protein expression at the oocyte surface by antibody binding to a FLAG epitope showed that the effect of trypsin was not accompanied by an increase in the channel protein density, indicating that proteolysis modified the activity of the channel present at the oocyte surface rather than the cell surface expression. At the single channel level, in the cell-attached mode, more active channels were observed in the patch when trypsin was present in the pipette, while no change in channel activity could be detected when trypsin was added to the bath solution around the patch pipette. We conclude that extracellular proteases are able to increase the open probability of the epithelial sodium channel by an effect that does not occur through activation of a G protein-coupled receptor, but rather through proteolysis of a protein that is either a constitutive part of the channel itself or closely associated with it.
Figures





Similar articles
-
cAMP sensitivity conferred to the epithelial Na+ channel by alpha-subunit cloned from guinea-pig colon.Pflugers Arch. 2000 Mar;439(5):579-87. doi: 10.1007/s004249900213. Pflugers Arch. 2000. PMID: 10764218
-
Influence of voltage and extracellular Na(+) on amiloride block and transport kinetics of rat epithelial Na(+) channel expressed in Xenopus oocytes.Pflugers Arch. 2002 Mar;443(5-6):882-91. doi: 10.1007/s00424-001-0773-x. Epub 2002 Jan 22. Pflugers Arch. 2002. PMID: 11889589
-
Cleavage in the {gamma}-subunit of the epithelial sodium channel (ENaC) plays an important role in the proteolytic activation of near-silent channels.J Physiol. 2008 Oct 1;586(19):4587-608. doi: 10.1113/jphysiol.2008.154435. Epub 2008 Jul 31. J Physiol. 2008. PMID: 18669538 Free PMC article.
-
ENaC activation by proteases.Acta Physiol (Oxf). 2022 May;235(1):e13811. doi: 10.1111/apha.13811. Epub 2022 Mar 21. Acta Physiol (Oxf). 2022. PMID: 35276025 Free PMC article. Review.
-
Epithelial sodium channel regulatory proteins identified by functional expression cloning.Kidney Int Suppl. 1998 Sep;67:S109-14. doi: 10.1046/j.1523-1755.1998.06721.x. Kidney Int Suppl. 1998. PMID: 9736264 Review.
Cited by
-
Acid-sensing ion channels in pathological conditions.Adv Exp Med Biol. 2013;961:419-31. doi: 10.1007/978-1-4614-4756-6_36. Adv Exp Med Biol. 2013. PMID: 23224900 Free PMC article. Review.
-
Cathepsin B is secreted apically from Xenopus 2F3 cells and cleaves the epithelial sodium channel (ENaC) to increase its activity.J Biol Chem. 2012 Aug 31;287(36):30073-83. doi: 10.1074/jbc.M111.338574. Epub 2012 Jul 10. J Biol Chem. 2012. PMID: 22782900 Free PMC article.
-
Effect of neutrophil elastase and its inhibitor EPI-hNE4 on transepithelial sodium transport across normal and cystic fibrosis human nasal epithelial cells.Respir Res. 2010 Oct 8;11(1):141. doi: 10.1186/1465-9921-11-141. Respir Res. 2010. PMID: 20932306 Free PMC article.
-
New role for plasmin in sodium homeostasis.Curr Opin Nephrol Hypertens. 2010 Jan;19(1):13-9. doi: 10.1097/MNH.0b013e3283330fb2. Curr Opin Nephrol Hypertens. 2010. PMID: 19864949 Free PMC article. Review.
-
Tissue kallikrein activation of the epithelial Na channel.Am J Physiol Renal Physiol. 2012 Aug 15;303(4):F540-50. doi: 10.1152/ajprenal.00133.2012. Epub 2012 May 23. Am J Physiol Renal Physiol. 2012. PMID: 22622459 Free PMC article.
References
-
- Canessa CM, Horisberger J-D, Rossier BC. Functional cloning of the epithelial sodium channel: relation with genes involved in neurodegeneration. Nature. 1993;361:467–470. - PubMed
-
- Canessa CM, Schild L, Buell G, Thorens B, Gautshi Y, Horisberger J-D, Rossier BC. The amiloride-sensitive epithelial sodium channel is made of three homologous subunits. Nature. 1994;367:463–467. - PubMed
-
- Durieux ME, Salafranca MN, Lynch KR. Trypsin induces Ca2+-activated Cl− currents in X. laevisoocytes. FEBS Lett. 1994;337:235–238. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

