Regulation of an inwardly rectifying ATP-sensitive K+ channel in the basolateral membrane of renal proximal tubule
- PMID: 9417142
- PMCID: PMC1887764
- DOI: 10.1085/jgp.111.1.161
Regulation of an inwardly rectifying ATP-sensitive K+ channel in the basolateral membrane of renal proximal tubule
Abstract
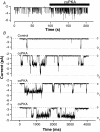
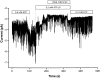
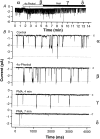
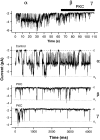
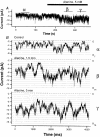
Functional coupling of Na+,K+-ATPase pump activity to a basolateral membrane (BLM) K+ conductance is crucial for sustaining transport in the proximal tubule. Apical sodium entry stimulates pump activity, lowering cytosolic [ATP], which in turn disinhibits ATP-sensitive K+ (KATP) channels. Opening of these KATP channels mediates hyperpolarization of the BLM that facilitates Na+ reabsorption and K+ recycling required for continued Na+,K+-ATPase pump turnover. Despite its physiological importance, little is known about the regulation of this channel. The present study focuses on the regulation of the BLM KATP channel by second messengers and protein kinases using membrane patches from dissociated, polarized Ambystoma proximal tubule cells. The channel is regulated by protein kinases A and C, but in opposing directions. The channel is activated by forskolin in cell-attached (c/a) patches, and by PKA in inside-out (i/o) membrane patches. However, phosphorylation by PKA is not sufficient to prevent channel rundown. In contrast, the channel is inhibited by phorbol ester in c/a patches, and PKC decreases channel activity (nPo) in i/o patches. The channel is pH sensitive, and lowering cytosolic pH reduces nPo. Increasing intracellular [Ca2+] ([Ca2+]i) in c/a patches decreases nPo, and this effect is direct since [Ca2+]i inhibits nPo with a Ki of approximately 170 nM in i/o patches. Membrane stretch and hypotonic swelling do not significantly affect channel behavior, but the channel appears to be regulated by the actin cytoskeleton. Finally, the activity of this BLM KATP channel is coupled to transcellular transport. In c/a patches, maneuvers that inhibit turnover of the Na+,K+-ATPase pump reduce nPo, presumably due to a rise in intracellular [ATP], although the associated cell depolarization cannot be ruled out as the possible cause. Conversely, stimulation of transport (and thus pump turnover) leads to increases in nPo, presumably due to a fall in intracellular [ATP]. These results show that the inwardly rectifying KATP channel in the BLM of the proximal tubule is a key element in the feedback system that links cellular metabolism with transport activity. We conclude that coupling of this KATP channel to the activity of the Na+,K+-ATPase pump is a mechanism by which steady state NaCl reabsorption in the proximal tubule may be maintained.
Figures

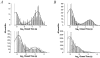







References
-
- Allard B, Lazdunski M. Nucleotide diphosphates activate the ATP-sensitive potassium channel in mouse skeletal muscle. Pflügers Arch. 1992;422:185–192. - PubMed
-
- Ashcroft SJ, Ashcroft FM. Properties and functions of ATP-sensitive K-channels. Cell Signal. 1990;2:197–214. - PubMed
-
- Beck JS, Hurst AM, Lapointe JY, Laprade R. Regulation of basolateral K channels in proximal tubule studied during continuous microperfusion. Am J Physiol. 1993;264:F496–F501. - PubMed
-
- Beck JS, Laprade R, Lapointe JY. Coupling between transepithelial Na transport and basolateral K conductance in renal proximal tubule [editorial] Am J Physiol. 1994;266:F517–F527. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

