A long-lived, highly luminescent Re(I) metal-ligand complex as a biomolecular probe
- PMID: 9417774
- PMCID: PMC6915065
- DOI: 10.1006/abio.1997.2413
A long-lived, highly luminescent Re(I) metal-ligand complex as a biomolecular probe
Abstract
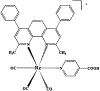
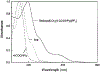
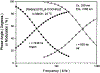
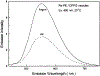
A highly luminescent rhenium (I) metal-ligand complex [Re(bcp)(CO)3(4-COOHPy)](ClO4), where bcp is 2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline and 4-COOHPy is isonicotinic acid, has been synthesized and characterized. High quantum yields (> 0.5) and long excited-state lifetimes (0.3-10 micronseconds) in fluid solutions at room temperature were found for this complex, with remarkable emission sensitivity to microenvironment. This compound also displays highly polarized emission with a maximum anisotropy near 0.3 in the absence of rotational diffusion. This Re complex was conjugated to several biomolecules, including the proteins human serum albumin and bovine immunoglobulin G, as well as an amine-containing lipid. When bound to a protein or lipid, the decay time is near 3 microseconds and the quantum yield is approximately 0.12 in aqueous oxygenated solution at room temperature. This compound's unique spectral properties along with its conjugatability allowed us to utilize it as biomolecular probe in a variety of environments.
Figures






Similar articles
-
Use of a long-lifetime Re(I) complex in fluorescence polarization immunoassays of high-molecular-weight analytes.Anal Chem. 1998 Feb 1;70(3):632-7. doi: 10.1021/ac970827k. Anal Chem. 1998. PMID: 9470490 Free PMC article.
-
Synthesis and spectral characterization of a thiol-reactive long-lifetime Ru(II) complex.Anal Biochem. 1997 Sep 5;251(2):241-5. doi: 10.1006/abio.1997.2253. Anal Biochem. 1997. PMID: 9299022 Free PMC article.
-
Synthesis and spectral characterization of a long-lifetime osmium (II) metal-ligand complex: a conjugatable red dye for applications in biophysics.Biophys Chem. 1999 Aug 30;80(3):143-51. doi: 10.1016/s0301-4622(99)00069-1. Biophys Chem. 1999. PMID: 10483708 Free PMC article.
-
Long-lifetime lipid rhenium metal-ligand complex for probing membrane dynamics on the microsecond timescale.Chem Phys Lipids. 1999 May;99(1):1-9. doi: 10.1016/s0009-3084(99)00002-x. Chem Phys Lipids. 1999. PMID: 10377961 Free PMC article.
-
Applications of luminescent inorganic and organometallic transition metal complexes as biomolecular and cellular probes.Dalton Trans. 2012 May 28;41(20):6021-47. doi: 10.1039/c2dt11892k. Epub 2012 Jan 13. Dalton Trans. 2012. PMID: 22241514 Review.
Cited by
-
Background suppression in frequency-domain fluorometry.Anal Biochem. 2000 Jan 1;277(1):74-85. doi: 10.1006/abio.1999.4346. Anal Biochem. 2000. PMID: 10610691 Free PMC article.
-
Glucose sensor for low-cost lifetime-based sensing using a genetically engineered protein.Anal Biochem. 1999 Feb 1;267(1):114-20. doi: 10.1006/abio.1998.2974. Anal Biochem. 1999. PMID: 9918662 Free PMC article.
-
Synthesis and characterization of novel rhenium(I) complexes towards potential biological imaging applications.Chem Cent J. 2016 Nov 25;10:71. doi: 10.1186/s13065-016-0218-4. eCollection 2016. Chem Cent J. 2016. PMID: 27942267 Free PMC article.
-
Photophysical studies of bioconjugated ruthenium metal-ligand complexes incorporated in phospholipid membrane bilayers.Inorg Chem. 2013 Oct 7;52(19):10835-45. doi: 10.1021/ic400706u. Epub 2013 Sep 24. Inorg Chem. 2013. PMID: 24063694 Free PMC article.
-
Low-frequency modulation sensors using nanosecond fluorophores.Anal Chem. 1998 Dec 15;70(24):5115-21. doi: 10.1021/ac980876c. Anal Chem. 1998. PMID: 9868909 Free PMC article.
References
-
- Seiler M, Durr H, Willner I, Joselevich E, Doron A, and Stoddart JF (1994) J. Am. Chem. Soc 116, 3399–3404.
-
- Juris A, and Balzani V (1988) Coord. Chem. Rev 84, 85–277.
-
- Kalyanasundaram K (1992) Photochemistry of Polypridine and Porphyrin Complexes, Academic Press, San Diego.
-
- Balzani V, and Scandola F (1991) Supramolecular Photochemistry, Ellis Horwood, Chichester/New York.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

