Structural determinants of SHP-2 function and specificity in Xenopus mesoderm induction
- PMID: 9418864
- PMCID: PMC121471
- DOI: 10.1128/MCB.18.1.161
Structural determinants of SHP-2 function and specificity in Xenopus mesoderm induction
Abstract
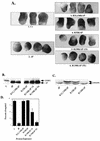
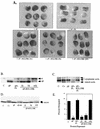
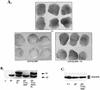
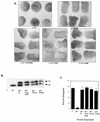
SHP-2 is a positive component of many receptor tyrosine kinase signaling pathways. The related protein-tyrosine phosphatase (PTP) SHP-1 usually acts as a negative regulator. The precise domains utilized by SHP-2 to transmit positive signals in vivo and the basis for specificity between SHP-1 and SHP-2 are not clear. In Xenopus, SHP-2 is required for mesoderm induction and completion of gastrulation. We investigated the effects of SHP-2 mutants and SHP-2/SHP-1 chimeras on basic fibroblast growth factor-induced mesoderm induction. Both SH2 domains and the PTP domain are required for normal SHP-2 function in this pathway. The N-terminal SH2 domain is absolutely required, whereas the C-terminal SH2 contributes to wild-type function. The C-terminal tyrosyl phosphorylation sites and proline-rich region are dispensable, arguing against adapter models of SHP-2 function. Although the SH2 domains contribute to SHP-2 specificity, studies of SHP chimeras reveal that substantial specificity resides in the PTP domain. Thus, PTP domains exhibit biologically relevant specificity in vivo, and noncatalytic and catalytic domains of PTPs contribute to specificity in a combinatorial fashion.
Figures


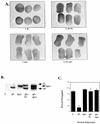


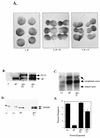
Similar articles
-
Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2.EMBO J. 1997 May 1;16(9):2352-64. doi: 10.1093/emboj/16.9.2352. EMBO J. 1997. PMID: 9171349 Free PMC article.
-
Src homology region 2 (SH2) domain-containing phosphatase-1 dephosphorylates B cell linker protein/SH2 domain leukocyte protein of 65 kDa and selectively regulates c-Jun NH2-terminal kinase activation in B cells.J Immunol. 2000 Aug 1;165(3):1344-51. doi: 10.4049/jimmunol.165.3.1344. J Immunol. 2000. PMID: 10903736
-
Specificity of the SH2 domains of SHP-1 in the interaction with the immunoreceptor tyrosine-based inhibitory motif-bearing receptor gp49B.J Immunol. 1999 Feb 1;162(3):1318-23. J Immunol. 1999. PMID: 9973385
-
Shp-2 tyrosine phosphatase: signaling one cell or many.Exp Cell Res. 1999 Nov 25;253(1):47-54. doi: 10.1006/excr.1999.4668. Exp Cell Res. 1999. PMID: 10579910 Review.
-
Regulation of B cell signal transduction by SH2-containing protein-tyrosine phosphatases.Semin Immunol. 1998 Aug;10(4):329-47. doi: 10.1006/smim.1998.0125. Semin Immunol. 1998. PMID: 9695189 Review.
Cited by
-
Protein Tyrosine Phosphatase SHP-2 (PTPN11) in Hematopoiesis and Leukemogenesis.J Signal Transduct. 2011;2011:195239. doi: 10.1155/2011/195239. Epub 2011 Jun 7. J Signal Transduct. 2011. PMID: 21799948 Free PMC article.
-
Inhibition of SHP2 and SHP1 Protein Tyrosine Phosphatase Activity by Chemically Induced Dimerization.ACS Omega. 2022 Apr 11;7(16):14180-14188. doi: 10.1021/acsomega.2c00780. eCollection 2022 Apr 26. ACS Omega. 2022. PMID: 35559188 Free PMC article.
-
Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses.J Cell Biol. 2000 Jun 26;149(7):1419-32. doi: 10.1083/jcb.149.7.1419. J Cell Biol. 2000. PMID: 10871282 Free PMC article.
-
Physiological signaling specificity by protein tyrosine phosphatases.Physiology (Bethesda). 2009 Oct;24:281-9. doi: 10.1152/physiol.00017.2009. Physiology (Bethesda). 2009. PMID: 19815854 Free PMC article. Review.
-
Noonan syndrome/leukemia-associated gain-of-function mutations in SHP-2 phosphatase (PTPN11) enhance cell migration and angiogenesis.J Biol Chem. 2009 Jan 9;284(2):913-20. doi: 10.1074/jbc.M804129200. Epub 2008 Nov 13. J Biol Chem. 2009. PMID: 19008228 Free PMC article.
References
-
- Allard J D, Chang H C, Herbst R, McNeill H, Simon M A. The SH2-containing tyrosine phosphatase corkscrew is required during signaling by sevenless, Ras1 and Raf. Development. 1996;122:1137–1146. - PubMed
-
- Amaya E, Musci T J, Kirschner M W. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991;66:257–260. - PubMed
-
- Amaya E, Stein P A, Musci T J, Kirschner M W. FGF signalling in the early specification of mesoderm in Xenopus. Development. 1993;118:477–487. - PubMed
-
- Aroian R V, Koga M, Mendel J E, Ohshima Y, Sternberg P W. The let-23 gene necessary for Caenorhabditis elegans vulval induction encodes a tyrosine kinase of the EGF receptor subfamily. Nature. 1990;348:693–699. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
