A novel mre11 mutation impairs processing of double-strand breaks of DNA during both mitosis and meiosis
- PMID: 9418873
- PMCID: PMC121488
- DOI: 10.1128/MCB.18.1.260
A novel mre11 mutation impairs processing of double-strand breaks of DNA during both mitosis and meiosis
Abstract
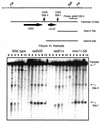
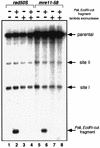
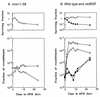
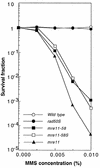
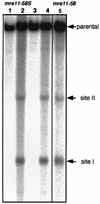
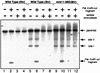
Using complementation tests and nucleotide sequencing, we showed that the rad58-4 mutation was an allele of the MRE11 gene and have renamed the mutation mre11-58. Two amino acid changes from the wild-type sequence were identified; one is located at a conserved site of a phosphodiesterase motif, and the other is a homologous amino acid change at a nonconserved site. Unlike mre11 null mutations, the mre11-58 mutation allowed meiosis-specific double-strand DNA breaks (DSBs) to form at recombination hot spots but failed to process those breaks. DSB ends of this mutant were resistant to lambda exonuclease treatment. These phenotypes are similar to those of rad50S mutants. In contrast to rad50S, however, mre11-58 was highly sensitive to methyl methanesulfonate treatment. DSB end processing induced by HO endonuclease was suppressed in both mre11-58 and the mre11 disruption mutant. We constructed a new mre11 mutant that contains only the phosphodiesterase motif mutation of the Mre11-58 protein and named it mre11-58S. This mutant showed the same phenotypes observed in mre11-58, suggesting that the phosphodiesterase consensus sequence is important for nucleolytic processing of DSB ends during both mitosis and meiosis.
Figures

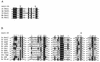

References
-
- Alani E, Padmore R, Kleckner N. Analysis of wild-type and rad50 mutants of yeast suggests an intimate relationship between meiotic chromosome synapsis and recombination. Cell. 1990;61:419–436. - PubMed
-
- Baum B. Mre11 and S. pombe Rad32 are phosphoesterases. Yeast Update Newsl. 1995;3.7:1.
-
- Bergerat A, de Massy B, Gadelle D, Varoutas P-C, Nicolas A, Forterre P. An atypical topoisomerase II from archaea with implications for meioric recombination. Nature. 1997;386:414–417. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
