A subunit of the anaphase-promoting complex is a centromere-associated protein in mammalian cells
- PMID: 9418894
- PMCID: PMC121516
- DOI: 10.1128/MCB.18.1.468
A subunit of the anaphase-promoting complex is a centromere-associated protein in mammalian cells
Abstract
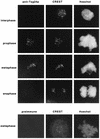
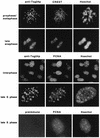
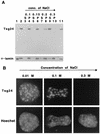
Sister chromatids in early mitotic cells are held together mainly by interactions between centromeres. The separation of sister chromatids at the transition between the metaphase and the anaphase stages of mitosis depends on the anaphase-promoting complex (APC), a 20S ubiquitin-ligase complex that targets proteins for destruction. A subunit of the APC, called APC-alpha in Xenopus (and whose homologs are APC-1, Cut4, BIME, and Tsg24), has recently been identified and shown to be required for entry into anaphase. We now show that the mammalian APC-alpha homolog, Tsg24, is a centromere-associated protein. While this protein is detected only during the prophase to the anaphase stages of mitosis in Chinese hamster cells, it is constitutively associated with the centromeres in murine cells. We show that there are two forms of this protein in mammalian cells, a soluble form associated with other components of the APC and a centromere-bound form. We also show that both the Tsg24 protein and the Cdc27 protein, another APC component, are bound to isolated mitotic chromosomes. These results therefore support a model in which the APC by ubiquitination of a centromere protein regulates the sister chromatid separation process.
Figures


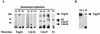
References
-
- Ault J G, Rieder C L. Centrosome and kinetochore movement during mitosis. Curr Opin Cell Biol. 1994;6:41–49. - PubMed
-
- Bhat M A, Philp A V, Glover D M, Bellen H J. Chromatid segregation at anaphase requires the barren product, a novel chromosome-associated protein that interacts with topoisomerase II. Cell. 1996;87:1103–1114. - PubMed
-
- Bickel S E, Orr-Weaver T L. Holding chromatids together to ensure they go their separate ways. Bioessays. 1996;18:293–300. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
