Requirement for phospholipase C-gamma1 enzymatic activity in growth factor-induced mitogenesis
- PMID: 9418905
- PMCID: PMC121526
- DOI: 10.1128/MCB.18.1.590
Requirement for phospholipase C-gamma1 enzymatic activity in growth factor-induced mitogenesis
Abstract
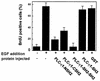
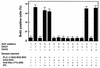
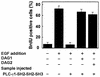
The cytoplasmic regions of the receptors for epidermal growth factor (EGF) and platelet-derived growth factor (PDGF) bind and activate phospholipase C-gamma1 (PLC-gamma1) and other signaling proteins in response to ligand binding outside the cell. Receptor binding by PLC-gamma1 is a function of its SH2 domains and is required for growth factor-induced cell cycle progression into the S phase. Microinjection into MDCK epithelial cells and NIH 3T3 fibroblasts of a polypeptide corresponding to the noncatalytic SH2-SH2-SH3 domains of PLC-gamma1 (PLC-gamma1 SH2-SH2-SH3) blocked growth factor-induced S-phase entry. Treatment of cells with diacylglycerol (DAG) or DAG and microinjected inositol-1,4,5-triphosphate (IP3), the products of activated PLC-gamma1, did not stimulate cellular DNA synthesis by themselves but did suppress the inhibitory effects of the PLC-gamma1 SH2-SH2-SH3 polypeptide but not the cell cycle block imposed by inhibition of the adapter protein Grb2 or p21 Ras. Two c-fos serum response element (SRE)-chloramphenicol acetyltransferase (CAT) reporter plasmids, a wild-type version, wtSRE-CAT, and a mutant, pm18, were used to investigate the function of PLC-gamma1 in EGF- and PDGF-induced mitogenesis. wtSRE-CAT responds to both protein kinase C (PKC)-dependent and -independent signals, while the mutant, pm18, responds only to PKC-independent signals. Microinjection of the dominant-negative PLC-gamma1 SH2-SH2-SH3 polypeptide greatly reduced the responses of wtSRE-CAT to EGF stimulation in MDCK cells and to PDGF stimulation in NIH 3T3 cells but had no effect on the responses of mutant pm18. These results indicate that in addition to Grb2-mediated activation of Ras, PLC-gamma1-mediated DAG production is required for EGF- and PDGF-induced S-phase entry and gene expression, possibly through activation of PKC.
Figures




Similar articles
-
Differential roles of the Src homology 2 domains of phospholipase C-gamma1 (PLC-gamma1) in platelet-derived growth factor-induced activation of PLC-gamma1 in intact cells.J Biol Chem. 2000 Mar 3;275(9):6411-6. doi: 10.1074/jbc.275.9.6411. J Biol Chem. 2000. PMID: 10692443
-
EGF-dependent association of phospholipase C-gamma1 with c-Cbl.Exp Cell Res. 2002 Jul 1;277(1):86-94. doi: 10.1006/excr.2002.5545. Exp Cell Res. 2002. PMID: 12061819
-
Physiological requirement for both SH2 domains for phospholipase C-gamma1 function and interaction with platelet-derived growth factor receptors.Mol Cell Biol. 1999 Jul;19(7):4961-70. doi: 10.1128/MCB.19.7.4961. Mol Cell Biol. 1999. PMID: 10373546 Free PMC article.
-
The mechanism of phospholipase C-gamma1 regulation.Exp Mol Med. 2000 Sep 30;32(3):101-9. doi: 10.1038/emm.2000.18. Exp Mol Med. 2000. PMID: 11048639 Review.
-
Upregulation of Phospholipase C Gene Expression Due to Norepinephrine-Induced Hypertrophic Response.Cells. 2022 Aug 11;11(16):2488. doi: 10.3390/cells11162488. Cells. 2022. PMID: 36010565 Free PMC article. Review.
Cited by
-
Diacylglycerol kinase zeta regulates Ras activation by a novel mechanism.J Cell Biol. 2001 Mar 19;152(6):1135-43. doi: 10.1083/jcb.152.6.1135. J Cell Biol. 2001. PMID: 11257115 Free PMC article.
-
Oxidative stress mediates the disruption of airway epithelial tight junctions through a TRPM2-PLCγ1-PKCα signaling pathway.Int J Mol Sci. 2013 Apr 29;14(5):9475-86. doi: 10.3390/ijms14059475. Int J Mol Sci. 2013. PMID: 23629676 Free PMC article.
-
Fibroblast growth factor acts upon the transcription of phospholipase C genes in human umbilical vein endothelial cells.Mol Cell Biochem. 2014 Mar;388(1-2):51-9. doi: 10.1007/s11010-013-1898-x. Epub 2013 Nov 16. Mol Cell Biochem. 2014. PMID: 24242047
-
Sts-2 is a phosphatase that negatively regulates zeta-associated protein (ZAP)-70 and T cell receptor signaling pathways.J Biol Chem. 2011 May 6;286(18):15943-54. doi: 10.1074/jbc.M110.177634. Epub 2011 Mar 10. J Biol Chem. 2011. PMID: 21393235 Free PMC article.
-
Phospholipases of mineralization competent cells and matrix vesicles: roles in physiological and pathological mineralizations.Int J Mol Sci. 2013 Mar 1;14(3):5036-129. doi: 10.3390/ijms14035036. Int J Mol Sci. 2013. PMID: 23455471 Free PMC article.
References
-
- Anderson D, Koch C A, Grey L, Ellis C, Moran M F, Pawson T. Binding of SH2 domains of phospholipase Cγ1, GAP, and Src to activated growth factor receptors. Science. 1990;250:979–982. - PubMed
-
- Bar-Sagi D, Rotin D, Batzer A, Mandiyan V, Schlessinger J. SH3 domains direct cellular localization of signaling molecules. Cell. 1993;74:83–91. - PubMed
-
- Gilman M Z. The c-fos serum response element responds to protein kinase C-dependent and -independent signals but not to cyclic AMP. Genes Dev. 1988;2:394–402. - PubMed
-
- Gout I, Dhand R, Hiles I D, Fry M J, Panayotou G, Das P, Truong O, Totty N F, Hsuan J, Booker G W. The GTPase dynamin binds to and is activated by a subset of SH3 domains. Cell. 1993;75:25–36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
