Sphingoid base 1-phosphate phosphatase: a key regulator of sphingolipid metabolism and stress response
- PMID: 9419344
- PMCID: PMC18156
- DOI: 10.1073/pnas.95.1.150
Sphingoid base 1-phosphate phosphatase: a key regulator of sphingolipid metabolism and stress response
Abstract
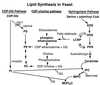
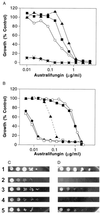
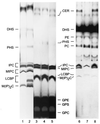
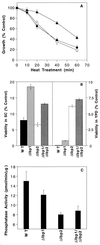
The sphingolipid metabolites ceramide and sphingosine-1-phosphate are second messengers with opposing roles in mammalian cell growth arrest and survival; their relative cellular level has been proposed to be a rheostat that determines the fate of cells. This report demonstrates that this rheostat is an evolutionarily conserved stress-regulatory mechanism that influences growth and survival of yeast. Although the role of sphingosine-1-phosphate in yeast was not previously examined, accumulation of ceramide has been shown to induce G1 arrest and cell death. We now have identified a gene in Saccharomyces cerevisiae, LBP1, that regulates the levels of phosphorylated sphingoid bases and ceramide. LBP1 was cloned from a yeast mutant that accumulated phosphorylated long-chain sphingoid bases and diverted sphingoid base intermediates from sphingolipid pathways to glycerophospholipid biosynthesis. LBP1 and its homolog, LBP2, encode very hydrophobic proteins that contain a novel-conserved sequence motif for lipid phosphatases, and both have long-chain sphingoid base phosphate phosphatase activity. In vitro characterization of Lbp1p shows that this phosphatase is Mg2+-independent with high specificity for phosphorylated long-chain bases, phytosphingosine and sphingosine. The deletion of LBP1 results in the accumulation of phosphorylated long-chain sphingoid bases and reduced ceramide levels. Moreover, deletion of LBP1 and LBP2 results in dramatically enhanced survival upon severe heat shock. Thus, these phosphatases play a previously unappreciated role in regulating ceramide and phosphorylated sphingoid base levels in yeast, and they modulate stress responses through sphingolipid metabolites in a manner that is reminiscent of their effects on mammalian cells.
Figures

Similar articles
-
Molecular cloning and characterization of a lipid phosphohydrolase that degrades sphingosine-1- phosphate and induces cell death.Proc Natl Acad Sci U S A. 2000 Jul 5;97(14):7859-64. doi: 10.1073/pnas.120146897. Proc Natl Acad Sci U S A. 2000. PMID: 10859351 Free PMC article.
-
Metabolism and selected functions of sphingolipids in the yeast Saccharomyces cerevisiae.Biochim Biophys Acta. 1999 Jun 10;1438(3):305-21. doi: 10.1016/s1388-1981(99)00068-2. Biochim Biophys Acta. 1999. PMID: 10366774 Review.
-
Sphingosine-1-phosphate and lipid phosphohydrolases.Biochim Biophys Acta. 2002 May 23;1582(1-3):8-17. doi: 10.1016/s1388-1981(02)00132-4. Biochim Biophys Acta. 2002. PMID: 12069805 Review.
-
Role for de novo sphingoid base biosynthesis in the heat-induced transient cell cycle arrest of Saccharomyces cerevisiae.J Biol Chem. 2001 Mar 16;276(11):8574-81. doi: 10.1074/jbc.M007425200. Epub 2000 Oct 30. J Biol Chem. 2001. PMID: 11056159
-
Yeast sphingosine-1-phosphate phosphatases: assay, expression, deletion, purification, and cellular localization by GFP tagging.Methods Enzymol. 2000;311:223-32. doi: 10.1016/s0076-6879(00)11085-7. Methods Enzymol. 2000. PMID: 10563329
Cited by
-
SVF1 regulates cell survival by affecting sphingolipid metabolism in Saccharomyces cerevisiae.Genetics. 2007 Jan;175(1):65-76. doi: 10.1534/genetics.106.064527. Epub 2006 Oct 22. Genetics. 2007. PMID: 17057230 Free PMC article.
-
Ceramide-Rubusoside Nanomicelles, a Potential Therapeutic Approach to Target Cancers Carrying p53 Missense Mutations.Mol Cancer Ther. 2020 Feb;19(2):564-574. doi: 10.1158/1535-7163.MCT-19-0366. Epub 2019 Oct 23. Mol Cancer Ther. 2020. PMID: 31645443 Free PMC article.
-
Ceramide Signaling and p53 Pathways.Adv Cancer Res. 2018;140:191-215. doi: 10.1016/bs.acr.2018.04.011. Epub 2018 Jun 1. Adv Cancer Res. 2018. PMID: 30060809 Free PMC article. Review.
-
Sli2 (Ypk1), a homologue of mammalian protein kinase SGK, is a downstream kinase in the sphingolipid-mediated signaling pathway of yeast.Mol Cell Biol. 2000 Jun;20(12):4411-9. doi: 10.1128/MCB.20.12.4411-4419.2000. Mol Cell Biol. 2000. PMID: 10825204 Free PMC article.
-
Increased protein kinase or decreased PP2A activity bypasses sphingoid base requirement in endocytosis.EMBO J. 2000 Jun 15;19(12):2834-44. doi: 10.1093/emboj/19.12.2834. EMBO J. 2000. PMID: 10856229 Free PMC article.
References
-
- Hannun Y A. Science. 1996;274:1855–1859. - PubMed
-
- Verheij M, Bose R, Lin S H, Yao B, Jarvis W D, Grant S, Birrer M J, Szabo E, Zon L I, Kyriakis J M, Haimovitz-Friedman A, Fuks Z, Kolesnick R. Nature (London) 1996;380:75–79. - PubMed
-
- Santana P, Pena L A, Haimovitz-Friedman A, Martin S, Green D, McLoughlin M, Cordon-Cardo C, Schuchman E H, Fuks Z, Kolesnick R. Cell. 1996;86:189–199. - PubMed
-
- Adam-Klages S, Adam D, Wiegmann K, Struve S, Kolanus W, Schneider-Mergener J, Kronke M. Cell. 1996;86:937–947. - PubMed
-
- Merrill A H, Jr, Schmelz E-M, Dillehay D L, Spiegel S, Shayman J A, Schroeder J J, Riley R T, Wang E. Toxicol Appl Pharmacol. 1997;142:208–225. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

