Hexamethylene bisacetamide induces programmed cell death (apoptosis) and down-regulates BCL-2 expression in human myeloma cells
- PMID: 9419346
- PMCID: PMC18160
- DOI: 10.1073/pnas.95.1.162
Hexamethylene bisacetamide induces programmed cell death (apoptosis) and down-regulates BCL-2 expression in human myeloma cells
Abstract
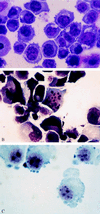
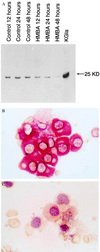
Multiple myeloma (MM) is a B cell malignancy characterized by the expansion of monoclonal Ig-secreting plasma cells with low proliferative activity. It is postulated that inhibition of physiologic cell death is an underlying factor in the pathophysiology of MM. The development of chemoresistance is a common feature in patients with MM. In the present studies, hexamethylene bisacetamide (HMBA), a hybrid polar compound that is a potent inducer of terminal differentiation of various transformed cells, is shown to inhibit the growth of several human myeloma cell lines (ARP-1, U266, and RPMI 8226), including doxorubicin-resistant RPMI 8226 variants that overexpress the multidrug-resistance gene, MDR-1, and its product, p-glycoprotein. In addition to growth arrest and suppression of clonogenicity, HMBA induces apoptosis both in freshly isolated human myeloma cells and in cell lines, as determined by morphologic alterations, cell cycle distribution and endonucleosomal DNA fragmentation. Further, HMBA decreases BCL-2 protein expression in myeloma cells within 12-48 hr. Overexpression of BCL-2 protein in ARP-1 cells confers resistance to HMBA-induced apoptosis. Taken together, these data suggest that HMBA is a potent inducer of apoptosis in human myeloma cells, which may act through suppressing the anti-apoptotic function of the bcl-2 gene. HMBA, and related hybrid polar compounds, may prove useful in the management of this presently incurable disease.
Figures


References
-
- Michaeli J, Rifkind R A, Marks P A. Cancer Chemother Biol Response Modifiers. 1992;13:287–307. - PubMed
-
- Williams G T. Cell. 1991;65:1097–1098. - PubMed
-
- Williams G T, Smith C A. Cell. 1993;74:777–779. - PubMed
-
- Richon V M, Russo P, Venta-Perez G, Cordon-Cardo C, Rifkind R A, Marks P A. Cancer Res. 1997;57:2789–2798. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

