A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes
- PMID: 9419363
- PMCID: PMC18193
- DOI: 10.1073/pnas.95.1.258
A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes
Abstract
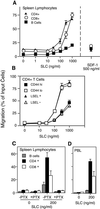
Preferential homing of naive lymphocytes to secondary lymphoid organs is thought to involve the action of chemokines, yet no chemokine has been shown to have either the expression pattern or the activities required to mediate this process. Here we show that a chemokine represented in the EST database, secondary lymphoid-tissue chemokine (SLC), is expressed in the high endothelial venules of lymph nodes and Peyer's patches, in the T cell areas of spleen, lymph nodes, and Peyer's patches, and in the lymphatic endothelium of multiple organs. SLC is a highly efficacious chemoattractant for lymphocytes with preferential activity toward naive T cells. Moreover, SLC induces firm adhesion of naive T lymphocytes via beta2 integrin binding to the counter receptor, intercellular adhesion molecule-1, a necessary step for lymphocyte recruitment. SLC is the first chemokine demonstrated to have the characteristics required to mediate homing of lymphocytes to secondary lymphoid organs. In addition, the expression of SLC in lymphatic endothelium suggests that the migration of lymphocytes from tissues into efferent lymphatics may be an active process mediated by this molecule.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

