Quantitative disassembly and reassembly of human papillomavirus type 11 viruslike particles in vitro
- PMID: 9420197
- PMCID: PMC109346
- DOI: 10.1128/JVI.72.1.32-41.1998
Quantitative disassembly and reassembly of human papillomavirus type 11 viruslike particles in vitro
Abstract
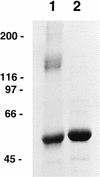
The human papillomavirus (HPV) capsid is primarily composed of a structural protein denoted L1, which forms both pentameric capsomeres and capsids composed of 72 capsomeres. The L1 protein alone is capable of self-assembly in vivo into capsidlike structures referred to as viruslike particles (VLPs). We have determined conditions for the quantitative disassembly of purified HPV-11 L1 VLPs to the level of capsomeres, demonstrating that disulfide bonds alone are essential to maintaining long-term HPV-11 L1 VLP structure at physiological ionic strength. The ionic strength of the disassembly reaction was also important, as increased NaCl concentrations inhibited disassembly. Conversely, chelation of cations had no effect on disassembly. Quantitative reassembly to a homogeneous population of 55-nm, 150S VLPs was reliably achieved by the re-formation of disulfide linkages following removal of reducing agent at near-neutral pH and moderate NaCl concentration. HPV-11 L1 VLPs could also be dissociated by treatment with carbonate buffer at pH 9.6, but VLPs could not be regenerated following carbonate treatment. When probed with conformationally sensitive and/or neutralizing monoclonal antibodies, both capsomeres generated by disulfide reduction of purified VLPs and reassembled VLPs formed from capsomeres upon removal of reducing agents exhibited epitopes found on the surface of authentic HPV-11 virions. Antisera raised against either purified VLP starting material or reassembled VLPs similarly neutralized infectious HPV-11 virions. The ability to disassemble and reassemble VLPs in vitro and in bulk allows basic features of capsid assembly to be studied and also opens the possibility of packaging selected exogenous compounds within the reassembled VLPs.
Figures





References
-
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

