The papillomavirus minor capsid protein, L2, induces localization of the major capsid protein, L1, and the viral transcription/replication protein, E2, to PML oncogenic domains
- PMID: 9420209
- PMCID: PMC109358
- DOI: 10.1128/JVI.72.1.142-150.1998
The papillomavirus minor capsid protein, L2, induces localization of the major capsid protein, L1, and the viral transcription/replication protein, E2, to PML oncogenic domains
Abstract
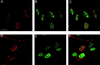
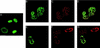
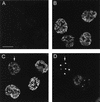
We have used immunofluorescent staining and confocal microscopy to examine the subcellular localization of structural and nonstructural bovine papillomavirus (BPV) proteins in cultured cells that produce infectious virions. When expressed separately, L1, the major capsid protein, showed a diffuse nuclear distribution while L2, the minor capsid protein, was found to localize to punctate nuclear regions identified as promonocytic leukemia protein (PML) oncogenic domains (PODs). Coexpression of L1 and L2 induced a relocation of L1 into the PODs, leading to the colocalization of L1 and L2. The effect of L2 expression on the distribution of the nonstructural viral proteins E1 and E2, which are required for maintenance of the genome and viral DNA synthesis, was also examined. The localization of the E1 protein was unaffected by L2 expression. However, the pattern of anti-E2 staining was dramatically altered in L2-expressing cells. Similar to L1, E2 was shifted from a dispersed nuclear locality into the PODs and colocalized with L2. The recruitment of full-length E2 by L2 occurred in the absence of other viral components. L2 was shown previously to be essential for the generation of infectious BPV. Our present results provide evidence for a role for L2 in the organization of virion components by recruiting them to a distinct nuclear domain. This L2-dependent colocalization probably serves as a mechanism to promote the assembly of papillomaviruses either by increasing the local concentration of virion constituents or by providing the physical architecture necessary for efficient packaging and assembly. The data also suggest a role for a nonstructural viral protein, E2, in virion assembly, specifically the recruitment of the viral genome to the sites of assembly, through its high-affinity interaction with specific sequences in the viral DNA.
Figures




References
-
- Androphy A J, Lowy D R, Schiller J T. Bovine papillomavirus E2 trans activating gene product binds to specific sites in papillomavirus DNA. Nature. 1987;325:70–73. - PubMed
-
- Boddy M N, Howe K, Etkin L D, Solomon E, Freemont P S. Pic 1, a novel ubiquitin-like protein which interacts with the PML component of a multiprotein complex that is disrupted in acute promyelocytic leukemia. Oncogene. 1996;13:971–982. - PubMed
-
- Carrasco L. The inhibition of cell functions after viral infection: a proposed general mechanism. FEBS Lett. 1977;76:11–15. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

