The N terminus of rotavirus VP2 is necessary for encapsidation of VP1 and VP3
- PMID: 9420216
- PMCID: PMC109365
- DOI: 10.1128/JVI.72.1.201-208.1998
The N terminus of rotavirus VP2 is necessary for encapsidation of VP1 and VP3
Abstract
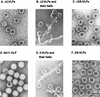
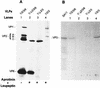
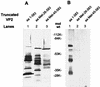
The innermost core of rotavirus is composed of VP2, which forms a protein layer that surrounds the two minor proteins VP1 and VP3, and the genome of 11 segments of double-stranded RNA. This inner core layer surrounded by VP6, the major capsid protein, constitutes double-layered particles that are transcriptionally active. Each gene encoding a structural protein of double-layered particles has been cloned into baculovirus recombinants and expressed in insect cells. Previously, we showed that coexpression of different combinations of the structural proteins of rotavirus double-layered particles results in the formation of virus-like particles (VLPs), and each VLP containing VP1, the presumed RNA-dependent RNA polymerase, possesses replicase activity as assayed in an in vitro template-dependent assay system (C. Q.-Y. Zeng, M. J. Wentz, J. Cohen, M. E. Estes, and R. F. Ramig, J. Virol. 70:2736-2742, 1996). This work reports construction and characterization of VLPs containing a truncated VP2 (VPdelta2, containing amino acids [aa] Met-93 to 880). Expression of VPdelta2 alone resulted in the formation of single-layered delta2-VLPs. Coexpression of VPdelta2 with VP6 produced double-layered delta2/6-VLPs. VLPs formed by coexpression of VPdelta2 and VP1 or VP3, or both VP1 and VP3, resulted in the formation of VLPs lacking both VP1 and VP3. The presence of VP6 with VPdelta2 did not result in encapsidation of VP1 and VP3. To determine the domain of VP2 required for binding VP1, far-Western blot analyses using a series of truncated VP2 constructs were performed to test their ability to bind VP1. These analyses showed that (i) full-length VP2 (aa 1 to 880) binds to VP1, (ii) any N-terminal truncation lacking aa 1 to 25 fails to bind VP1, and (iii) a C-terminal 296-aa truncated VP2 construct (aa 1 to 583) maintains the ability to bind VP1. These analyses indicate that the N terminus of rotavirus VP2 is necessary for the encapsidation of VP1 and VP3.
Figures




Similar articles
-
Characterization and replicase activity of double-layered and single-layered rotavirus-like particles expressed from baculovirus recombinants.J Virol. 1996 May;70(5):2736-42. doi: 10.1128/JVI.70.5.2736-2742.1996. J Virol. 1996. PMID: 8627747 Free PMC article.
-
Characterization of rotavirus VP2 particles.Virology. 1994 May 15;201(1):55-65. doi: 10.1006/viro.1994.1265. Virology. 1994. PMID: 8178489
-
Characterization of virus-like particles produced by the expression of rotavirus capsid proteins in insect cells.J Virol. 1994 Sep;68(9):5945-52. doi: 10.1128/JVI.68.9.5945-5952.1994. J Virol. 1994. PMID: 8057471 Free PMC article.
-
[Rotaviruses: structure and function of the principal polypeptides].Ann Rech Vet. 1989;20(4):431-42. Ann Rech Vet. 1989. PMID: 2559646 Review. French.
-
Nonstructural proteins involved in genome packaging and replication of rotaviruses and other members of the Reoviridae.Virus Res. 2004 Apr;101(1):57-66. doi: 10.1016/j.virusres.2003.12.006. Virus Res. 2004. PMID: 15010217 Review.
Cited by
-
Mechanism for coordinated RNA packaging and genome replication by rotavirus polymerase VP1.Structure. 2008 Nov 12;16(11):1678-88. doi: 10.1016/j.str.2008.09.006. Structure. 2008. PMID: 19000820 Free PMC article.
-
Group A Rotavirus VP1 Polymerase and VP2 Core Shell Proteins: Intergenotypic Sequence Variation and In Vitro Functional Compatibility.J Virol. 2019 Jan 4;93(2):e01642-18. doi: 10.1128/JVI.01642-18. Print 2019 Jan 15. J Virol. 2019. PMID: 30355692 Free PMC article.
-
Interaction between a Unique Minor Protein and a Major Capsid Protein of Bluetongue Virus Controls Virus Infectivity.J Virol. 2018 Jan 17;92(3):e01784-17. doi: 10.1128/JVI.01784-17. Print 2018 Feb 1. J Virol. 2018. PMID: 29142128 Free PMC article.
-
Mechanism of intraparticle synthesis of the rotavirus double-stranded RNA genome.J Biol Chem. 2010 Jun 11;285(24):18123-8. doi: 10.1074/jbc.R110.117671. Epub 2010 Mar 29. J Biol Chem. 2010. PMID: 20351108 Free PMC article.
-
Rotavirus RNA replication requires a single-stranded 3' end for efficient minus-strand synthesis.J Virol. 1998 Sep;72(9):7387-96. doi: 10.1128/JVI.72.9.7387-7396.1998. J Virol. 1998. PMID: 9696835 Free PMC article.
References
-
- Blacklow N R, Greenberg H B. Viral gastroenteritis. N Engl J Med. 1991;325:252–264. - PubMed
-
- Burroughs, M. H., S. E. Crawford, and M. K. Estes. Unpublished data.
-
- Chen D, Gombold J L, Ramig R F. Intracellular RNA synthesis directed by temperature-sensitive mutant of simian rotavirus SA11. Virology. 1990;178:143–151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

