The tripartite leader sequence of subgroup C adenovirus major late mRNAs can increase the efficiency of mRNA export
- PMID: 9420219
- PMCID: PMC109368
- DOI: 10.1128/JVI.72.1.225-235.1998
The tripartite leader sequence of subgroup C adenovirus major late mRNAs can increase the efficiency of mRNA export
Abstract
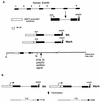
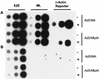
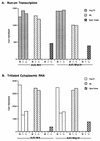
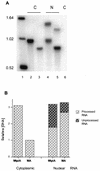
The subgroup C human adenoviruses induce selective export of newly synthesized viral mRNA from the nucleus to the cytoplasm, with concomitant inhibition of export of the majority of cellular mRNA species. Such posttranscriptional regulation of viral and cellular gene expression in infected cells requires viral E1B and E4 proteins. To facilitate the investigation of parameters that govern selective export in adenovirus-infected cells, we constructed a marked human beta-actin minigene under the control of the glucocorticoid-inducible enhancer-promoter of mouse mammary tumor virus and introduced it into the left end of the adenovirus type 5 (Ad5) genome. Transcription of this reporter gene (designated MA) as well as of a sibling, which differed only in the inclusion of a cDNA copy of the Ad2 major late tripartite leader sequence upstream of beta-actin sequences (termed MtplA), in recombinant virus-infected cells was strictly dependent on the addition of dexamethasone to the medium. When transcription of the MA gene was induced during the late phase of infection, newly synthesized MA RNA entered the cytoplasm. These transcripts, which contain no viral sequences, therefore reproduce the behavior of exceptional cellular mRNA species observed when transcription of their genes is activated during the late phase of infection (U.-C. Yang, W. Huang, and S. J. Flint, J. Virol. 70:4071-4080, 1996). Unexpectedly, however, higher concentrations of newly synthesized RNA accumulated in the cytoplasm when the tripartite leader sequence was present in the reporter RNA, despite equal rates of transcription of the two reporter genes. Examination of the partitioning of both newly synthesized and steady-state populations of MA and MtplA RNAs between nuclear and cytoplasmic compartments indicated that the tripartite leader sequence did not increase RNA stability in the cytoplasm. Comparison of nuclear and cytoplasmic reporter RNA species by Northern blotting, primer extension, and reverse transcription-PCR provided no evidence for altered processing induced by the tripartite leader sequence. We therefore conclude that the tripartite leader sequence, long known to facilitate the translation of mRNAs during the late phase of adenovirus infection, can also modulate mRNA export from the nucleus.
Figures

Similar articles
-
[Expression regulation of major late promoter tripartite leader sequence in adenovirus late infection].Zhonghua Yi Xue Za Zhi. 1998 Oct;78(10):733-7. Zhonghua Yi Xue Za Zhi. 1998. PMID: 11038823 Chinese.
-
mRNA export correlates with activation of transcription in human subgroup C adenovirus-infected cells.J Virol. 1996 Jun;70(6):4071-80. doi: 10.1128/JVI.70.6.4071-4080.1996. J Virol. 1996. PMID: 8648745 Free PMC article.
-
The adenovirus tripartite leader sequence can alter nuclear and cytoplasmic metabolism of a non-adenovirus mRNA within infected cells.Nucleic Acids Res. 1988 Mar 25;16(5):2247-62. doi: 10.1093/nar/16.5.2247. Nucleic Acids Res. 1988. PMID: 3357776 Free PMC article.
-
Regulation of mRNA production by the adenoviral E1B 55-kDa and E4 Orf6 proteins.Curr Top Microbiol Immunol. 2003;272:287-330. doi: 10.1007/978-3-662-05597-7_10. Curr Top Microbiol Immunol. 2003. PMID: 12747554 Review.
-
Release of viruses and viral DNA from nucleus to cytoplasm of HeLa cells at late stages of productive adenovirus infection as revealed by electron microscope in situ hybridization.Biol Cell. 1998 Jan;90(1):5-38. doi: 10.1016/s0248-4900(98)80230-x. Biol Cell. 1998. PMID: 9691424 Review.
Cited by
-
Adenovirus ubiquitin-protein ligase stimulates viral late mRNA nuclear export.J Virol. 2007 Jan;81(2):575-87. doi: 10.1128/JVI.01725-06. Epub 2006 Nov 1. J Virol. 2007. PMID: 17079297 Free PMC article.
-
The adenovirus L4-22K protein has distinct functions in the posttranscriptional regulation of gene expression and encapsidation of the viral genome.J Virol. 2013 Jul;87(13):7688-99. doi: 10.1128/JVI.00859-13. Epub 2013 May 1. J Virol. 2013. PMID: 23637408 Free PMC article.
-
Two novel adenovirus vector systems permitting regulated protein expression in gene transfer experiments.J Virol. 1998 Oct;72(10):8358-61. doi: 10.1128/JVI.72.10.8358-8361.1998. J Virol. 1998. PMID: 9733884 Free PMC article.
-
The tripartite leader sequence is required for ectopic expression of HAdV-B and HAdV-E E3 CR1 genes.Virology. 2017 May;505:139-147. doi: 10.1016/j.virol.2017.02.021. Epub 2017 Mar 1. Virology. 2017. PMID: 28259047 Free PMC article.
-
Unusual properties of adenovirus E2E transcription by RNA polymerase III.J Virol. 2003 Apr;77(7):4015-24. doi: 10.1128/jvi.77.7.4015-4024.2003. J Virol. 2003. PMID: 12634361 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

