Cells with high cyclophilin A content support replication of human immunodeficiency virus type 1 Gag mutants with decreased ability to incorporate cyclophilin A
- PMID: 9420228
- PMCID: PMC109377
- DOI: 10.1128/JVI.72.1.303-308.1998
Cells with high cyclophilin A content support replication of human immunodeficiency virus type 1 Gag mutants with decreased ability to incorporate cyclophilin A
Abstract
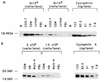
Gag polyprotein-mediated incorporation of cellular cyclophilin A (CyPA) into virions is essential for the formation of infectious human immunodeficiency virus type 1 (HIV-1) virions. Either a point mutation in Gag (P222A) or drugs which bind CyPA decrease virion incorporation of CyPA and interfere with HIV-1 replication. We have found that lymphoid cells varied greatly in their CyPA content and that cells with high CyPA content supported the replication of P222A HIV-1 Gag mutants. These experiments demonstrated that a higher cellular CyPA content of some cells was able to compensate for the decreased binding affinity of P222A mutant Gag for CyPA, allowing virus replication to occur.
Figures




References
-
- Bess J W, Jr, Gorelick R J, Bosche W J, Henderson L E, Arthur L O. Microvesicles are a source of contaminating cellular proteins found in purified HIV-1 preparations. Virology. 1997;230:134–144. - PubMed
-
- Billich A, Hammerschmid F, Peichl P, Wenger R, Zenke G, Quesniaux V, Rosenwirth B. Mode of action of SDZ NIM 811, a nonimmunosuppressive cyclosporin A analog with activity against human immunodeficiency virus (HIV) type 1: interference with protein-cyclophilin A interactions. J Virol. 1995;69:2451–2461. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

